Progressive, transgenerational changes in offspring phenotype and epigenotype following nutritional transition
- PMID: 22140567
- PMCID: PMC3227644
- DOI: 10.1371/journal.pone.0028282
Progressive, transgenerational changes in offspring phenotype and epigenotype following nutritional transition
Abstract
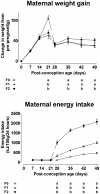
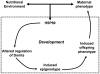
Induction of altered phenotypes during development in response to environmental input involves epigenetic changes. Phenotypic traits can be passed between generations by a variety of mechanisms, including direct transmission of epigenetic states or by induction of epigenetic marks de novo in each generation. To distinguish between these possibilities we measured epigenetic marks over four generations in rats exposed to a sustained environmental challenge. Dietary energy was increased by 25% at conception in F0 female rats and maintained at this level to generation F3. F0 dams showed higher pregnancy weight gain, but lower weight gain and food intake during lactation than F1 and F2 dams. On gestational day 8, fasting plasma glucose concentration was higher and β-hydroxybutyrate lower in F0 and F1 dams than F2 dams. This was accompanied by decreased phosphoenolpyruvate carboxykinase (PEPCK) and increased PPARα and carnitine palmitoyl transferase-1 mRNA expression. PEPCK mRNA expression was inversely related to the methylation of specific CpG dinucleotides in its promoter. DNA methyltransferase (Dnmt) 3a2, but not Dnmt1 or Dnmt3b, expression increased and methylation of its promoter decreased from F1 to F3 generations. These data suggest that the regulation of energy metabolism during pregnancy and lactation within a generation is influenced by the maternal phenotype in the preceding generation and the environment during the current pregnancy. The transgenerational effects on phenotype were associated with altered DNA methylation of specific genes in a manner consistent with induction de novo of epigenetic marks in each generation.
Conflict of interest statement
Figures




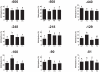
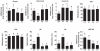
References
-
- Kimura M. Cambridge: Cambridge University Press; 1984. The Neutral Theory of Molecular Evolution.
-
- West-Eberhard MJ. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J Exp Zool B Mol Dev Evol. 2005;304:610–618. - PubMed
-
- Badyaev AV. Maternal effects as generators of evolutionary change: a reassessment. Ann N Y Acad Sci. 2008;1133:151–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

