Genetic control of the variable innate immune response to asymptomatic bacteriuria
- PMID: 22140570
- PMCID: PMC3225390
- DOI: 10.1371/journal.pone.0028289
Genetic control of the variable innate immune response to asymptomatic bacteriuria
Erratum in
- PLoS One. 2012;7(1):doi/10.1371/annotation/ab823a8e-01b1-48b8-8b2a-0993d805fc50. Hernández, Jenny Grönberg [corrected to Grönberg-Hernández, Jenny]
Abstract
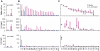
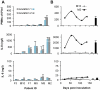
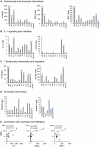
The severity of urinary tract infection (UTI) reflects the quality and magnitude of the host response. While strong local and systemic innate immune activation occurs in patients with acute pyelonephritis, the response to asymptomatic bacteriuria (ABU) is low. The immune response repertoire in ABU has not been characterized, due to the inherent problem to distinguish bacterial differences from host-determined variation. In this study, we investigated the host response to ABU and genetic variants affecting innate immune signaling and UTI susceptibility. Patients were subjected to therapeutic urinary tract inoculation with E. coli 83972 to ensure that they were exposed to the same E. coli strain. The innate immune response repertoire was characterized in urine samples, collected from each patient before and after inoculation with bacteria or PBS, if during the placebo arm of the study. Long-term E. coli 83972 ABU was established in 23 participants, who were followed for up to twelve months and the innate immune response was quantified in 233 urine samples. Neutrophil numbers increased in all but two patients and in an extended urine cytokine/chemokine analysis (31 proteins), the chemoattractants IL-8 and GRO-α, RANTES, Eotaxin-1 and MCP-1, the T cell chemoattractant and antibacterial peptide IP-10, inflammatory regulators IL-1-α and sIL-1RA and the T lymphocyte/dendritic cell product sIL-2Rα were detected and variably increased, compared to sterile samples. IL-6, which is associated with symptomatic UTI, remained low and numerous specific immune mediators were not detected. The patients were also genotyped for UTI-associated IRF3 and TLR4 promoter polymorphisms. Patients with ABU associated TLR4 polymorphisms had low neutrophil numbers, IL-6, IP-10, MCP-1 and sIL-2Rα concentrations. Patients with the ABU-associated IRF3 genotype had lower neutrophils, IL-6 and MCP-1 responses than the remaining group. The results suggest that the host-specific, low immune response to ABU mainly includes innate immune mediators and that host genetics directly influence the magnitude of this response.
Conflict of interest statement
Figures
Similar articles
-
Susceptibility to acute pyelonephritis or asymptomatic bacteriuria: host-pathogen interaction in urinary tract infections.Pediatr Nephrol. 2012 Nov;27(11):2017-2029. doi: 10.1007/s00467-011-2089-1. Epub 2012 Feb 12. Pediatr Nephrol. 2012. PMID: 22327887 Review.
-
Genetic variation of the human urinary tract innate immune response and asymptomatic bacteriuria in women.PLoS One. 2009 Dec 15;4(12):e8300. doi: 10.1371/journal.pone.0008300. PLoS One. 2009. PMID: 20016852 Free PMC article.
-
Toll-like receptor 4 promoter polymorphisms: common TLR4 variants may protect against severe urinary tract infection.PLoS One. 2010 May 20;5(5):e10734. doi: 10.1371/journal.pone.0010734. PLoS One. 2010. PMID: 20505764 Free PMC article.
-
Innate immunity and genetic determinants of urinary tract infection susceptibility.Curr Opin Infect Dis. 2015 Feb;28(1):88-96. doi: 10.1097/QCO.0000000000000127. Curr Opin Infect Dis. 2015. PMID: 25539411 Free PMC article. Review.
-
TLR- and CXCR1-dependent innate immunity: insights into the genetics of urinary tract infections.Eur J Clin Invest. 2008 Oct;38 Suppl 2:12-20. doi: 10.1111/j.1365-2362.2008.02004.x. Eur J Clin Invest. 2008. PMID: 18826477 Review.
Cited by
-
Bacterial control of host gene expression through RNA polymerase II.J Clin Invest. 2013 Jun;123(6):2366-79. doi: 10.1172/JCI66451. J Clin Invest. 2013. PMID: 23728172 Free PMC article.
-
Molecular analysis of asymptomatic bacteriuria Escherichia coli strain VR50 reveals adaptation to the urinary tract by gene acquisition.Infect Immun. 2015 May;83(5):1749-64. doi: 10.1128/IAI.02810-14. Epub 2015 Feb 9. Infect Immun. 2015. PMID: 25667270 Free PMC article.
-
The etiology and prevalence of urinary tract infection and asymptomatic bacteriuria in pregnant women in Iran: a systematic review and Meta-analysis.BMC Urol. 2019 May 30;19(1):43. doi: 10.1186/s12894-019-0454-8. BMC Urol. 2019. PMID: 31146773 Free PMC article.
-
Recurrent urinary tract infection genetic risk: a systematic review and gene network analysis.Int Urogynecol J. 2024 Feb;35(2):259-271. doi: 10.1007/s00192-023-05671-6. Epub 2023 Nov 2. Int Urogynecol J. 2024. PMID: 37917182
-
Active bacterial modification of the host environment through RNA polymerase II inhibition.J Clin Invest. 2021 Feb 15;131(4):e140333. doi: 10.1172/JCI140333. J Clin Invest. 2021. PMID: 33320835 Free PMC article.
References
-
- Otto G, Burdick M, Strieter R, Godaly G. Chemokine response to febrile urinary tract infection. Kidney Int. 2005;68:62–70. - PubMed
-
- Benson M, Jodal U, Andreasson A, Karlsson A, Rydberg J, et al. Interleukin 6 response to urinary tract infection in childhood. Pediatr Infect Dis J. 1994;13:612–616. - PubMed
-
- Hansson S, Martinell J, Stokland E, Jodal U. The natural history of bacteriuria in childhood. Infectious disease clinics of North America. 1997;11:499–512. - PubMed
-
- Lindberg U, Claesson I, Hanson LA, Jodal U. Asymptomatic bacteriuria in schoolgirls. I. Clinical and laboratory findings. Acta paediatrica Scandinavica. 1975;64:425–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

