Growth hormone promotes hair cell regeneration in the zebrafish (Danio rerio) inner ear following acoustic trauma
- PMID: 22140580
- PMCID: PMC3227666
- DOI: 10.1371/journal.pone.0028372
Growth hormone promotes hair cell regeneration in the zebrafish (Danio rerio) inner ear following acoustic trauma
Abstract
Background: Previous microarray analysis showed that growth hormone (GH) was significantly upregulated following acoustic trauma in the zebrafish (Danio rerio) ear suggesting that GH may play an important role in the process of auditory hair cell regeneration. Our objective was to examine the effects of exogenous and endogenous GH on zebrafish inner ear epithelia following acoustic trauma.
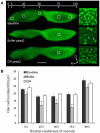
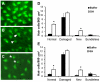
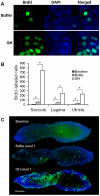
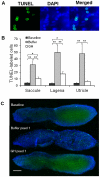
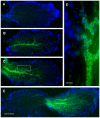
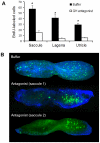
Methodology/principal findings: We induced auditory hair cell damage by exposing zebrafish to acoustic overstimulation. Fish were then injected intraperitoneally with either carp GH or buffer, and placed in a recovery tank for either one or two days. Phalloidin-, bromodeoxyuridine (BrdU)-, and TUNEL-labeling were used to examine hair cell densities, cell proliferation, and apoptosis, respectively. Two days post-trauma, saccular hair cell densities in GH-treated fish were similar to that of baseline controls, whereas buffer-injected fish showed significantly reduced densities of hair cell bundles. Cell proliferation was greater and apoptosis reduced in the saccules, lagenae, and utricles of GH-treated fish one day following trauma compared to controls. Fluorescent in situ hybridization (FISH) was used to examine the localization of GH mRNA in the zebrafish ear. At one day post-trauma, GH mRNA expression appeared to be localized perinuclearly around erythrocytes in the blood vessels of the inner ear epithelia. In order to examine the effects of endogenous GH on the process of cell proliferation in the ear, a GH antagonist was injected into zebrafish immediately following acoustic trauma, resulting in significantly decreased cell proliferation one day post-trauma in all three zebrafish inner ear end organs.
Conclusions/significance: Our results show that exogenous GH promotes post-trauma auditory hair cell regeneration in the zebrafish ear through stimulating proliferation and suppressing apoptosis, and that endogenous GH signals are present in the zebrafish ear during the process of auditory hair cell regeneration.
Conflict of interest statement
Figures
Similar articles
-
Cell proliferation follows acoustically-induced hair cell bundle loss in the zebrafish saccule.Hear Res. 2009 Jul;253(1-2):67-76. doi: 10.1016/j.heares.2009.03.008. Epub 2009 Mar 25. Hear Res. 2009. PMID: 19327392 Free PMC article.
-
Transcriptomic analysis of the zebrafish inner ear points to growth hormone mediated regeneration following acoustic trauma.BMC Neurosci. 2011 Sep 2;12:88. doi: 10.1186/1471-2202-12-88. BMC Neurosci. 2011. PMID: 21888654 Free PMC article.
-
Anatomical and functional recovery of the goldfish (Carassius auratus) ear following noise exposure.J Exp Biol. 2006 Nov;209(Pt 21):4193-202. doi: 10.1242/jeb.02490. J Exp Biol. 2006. PMID: 17050834
-
Functional recovery in the avian ear after hair cell regeneration.Audiol Neurootol. 1999 Nov-Dec;4(6):286-302. doi: 10.1159/000013853. Audiol Neurootol. 1999. PMID: 10516389 Review.
-
Hair cell regeneration in the inner ear: a review.Clin Otolaryngol Allied Sci. 2003 Feb;28(1):5-13. doi: 10.1046/j.1365-2273.2003.00658.x. Clin Otolaryngol Allied Sci. 2003. PMID: 12580872 Review.
Cited by
-
Hearing sensitivity differs between zebrafish lines used in auditory research.Hear Res. 2016 Nov;341:220-231. doi: 10.1016/j.heares.2016.09.004. Epub 2016 Sep 16. Hear Res. 2016. PMID: 27646864 Free PMC article.
-
Identification of a gene set to evaluate the potential effects of loud sounds from seismic surveys on the ears of fishes: a study with Salmo salar.J Fish Biol. 2014 Jun;84(6):1793-819. doi: 10.1111/jfb.12398. Epub 2014 May 9. J Fish Biol. 2014. PMID: 24814183 Free PMC article.
-
Insulin-like Growth Factor 1 Signaling in Mammalian Hearing.Genes (Basel). 2021 Sep 29;12(10):1553. doi: 10.3390/genes12101553. Genes (Basel). 2021. PMID: 34680948 Free PMC article. Review.
-
Effects of high intensity noise on the vestibular system in rats.Hear Res. 2016 May;335:118-127. doi: 10.1016/j.heares.2016.03.002. Epub 2016 Mar 10. Hear Res. 2016. PMID: 26970474 Free PMC article.
-
Transcriptomic Profiling Revealed Signaling Pathways Associated with the Spawning of Female Zebrafish under Cold Stress.Int J Mol Sci. 2022 Jul 6;23(14):7494. doi: 10.3390/ijms23147494. Int J Mol Sci. 2022. PMID: 35886843 Free PMC article.
References
-
- Löwenstein O. Functional anatomy of the vertebrate gravity receptor system. In: Gordon SA, Cohen MJ, editors. Gravity and the Organism. Chicago: University of Chicago Press; 1971. pp. 253–261.
-
- Hudspeth AJ. The cellular basis of hearing: the biophysics of hair cells. Science. 1985;230:745–752. - PubMed
-
- Fettiplace R, Ricci AJ. Mechanoelectrical transduction in auditory hair cells. In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate Hair Cells. New York: Springer Science+Business Media, Inc; 2006. pp. 154–203.
-
- Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am J Otol. 1994;15:28–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

