Epigenetic mechanisms of pulmonary hypertension
- PMID: 22140624
- PMCID: PMC3224426
- DOI: 10.4103/2045-8932.87300
Epigenetic mechanisms of pulmonary hypertension
Abstract
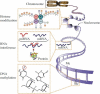
Epigenetics refers to changes in phenotype and gene expression that occur without alterations in DNA sequence. Epigenetic modifications of the genome can be acquired de novo and are potentially heritable. This review focuses on the emerging recognition of a role for epigenetics in the development of pulmonary arterial hypertension (PAH). Lessons learned from the epigenetics in cancer and neurodevelopmental diseases, such as Prader-Willi syndrome, can be applied to PAH. These syndromes suggest that there is substantial genetic and epigenetic cross-talk such that a single phenotype can result from a genetic cause, an epigenetic cause, or a combined abnormality. There are three major mechanisms of epigenetic regulation, including methylation of CpG islands, mediated by DNA methyltransferases, modification of histone proteins, and microRNAs. There is substantial interaction between these epigenetic mechanisms. Recently, it was discovered that there may be an epigenetic component to PAH. In PAH there is downregulation of superoxide dismutase 2 (SOD2) and normoxic activation of hypoxia inducible factor (HIF-1α). This decrease in SOD2 results from methylation of CpG islands in SOD2 by lung DNA methyltransferases. The partial silencing of SOD2 alters redox signaling, activates HIF-1α) and leads to excessive cell proliferation. The same hyperproliferative epigenetic abnormality occurs in cancer. These epigenetic abnormalities can be therapeutically reversed. Epigenetic mechanisms may mediate gene-environment interactions in PAH and explain the great variability in susceptibility to stimuli such as anorexigens, virus, and shunts. Epigenetics may be relevant to the female predisposition to PAH and the incomplete penetrance of BMPR2 mutations in familial PAH.
Keywords: CpG islands; DNA methyl transferases; histone acetylation; small inhibitor RNA; superoxide dismutase 2.
Conflict of interest statement
Figures



References
-
- Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–4. - PubMed
-
- Nagaoka T, Muramatsu M, Sato K, McMurtry I, Oka M, Fukuchi Y. Mild hypoxia causes severe pulmonary hypertension in fawn-hooded but not in Tester Moriyama rats. Respir Physiol. 2001;127:53–60. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

