17β-Oestradiol inhibits doxorubicin-induced apoptosis via block of the volume-sensitive Cl(-) current in rabbit articular chondrocytes
- PMID: 22142024
- PMCID: PMC3417499
- DOI: 10.1111/j.1476-5381.2011.01802.x
17β-Oestradiol inhibits doxorubicin-induced apoptosis via block of the volume-sensitive Cl(-) current in rabbit articular chondrocytes
Abstract
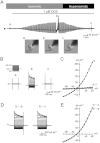
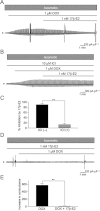
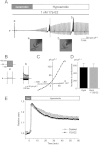
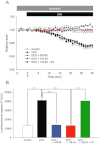
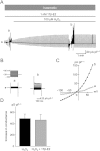
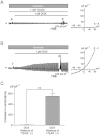
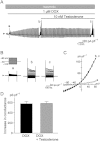
BACKGROUND AND PURPOSE Chondrocyte apoptosis contributes to disruption of cartilage integrity in osteoarthritis. Recent evidence suggested that the volume-sensitive organic osmolyte/anion channel [volume-sensitive (outwardly rectifying) Cl(-) current (I(Cl,vol) )] plays a functional role in the development of cell shrinkage associated with apoptosis (apoptotic volume decrease) in several cell types. In this study, we investigated the cellular effects of 17β-oestradiol on doxorubicin-induced apoptotic responses in rabbit articular chondrocytes. EXPERIMENTAL APPROACH Whole-cell membrane currents and cross-sectional area were measured from chondrocytes using a patch-clamp method and microscopic cell imaging, respectively. Caspase-3/7 activity was assayed as an index of apoptosis. KEY RESULTS Addition of doxorubicin (1 µM) to isosmotic bath solution rapidly activated the Cl(-) current with properties similar to those of I(Cl,vol) in chondrocytes. Doxorubicin also gradually decreased the cross-sectional area of chondrocytes, followed by enhanced caspase-3/7 activity; both of these responses were totally abolished by the I(Cl,vol) blocker DCPIB (20 µM). Pretreatment of chondrocytes with 17β-oestradiol (1 nM) for short (approximately 10 min) and long (24 h) periods almost completely prevented the doxorubicin-induced activation of I(Cl,vol) and subsequent elevation of caspase-3/7 activity. These effects of 17β-oestradiol were significantly attenuated by the oestrogen receptor blocker ICI 182780 (10 µM), as well as the phosphatidyl inositol-3-kinase (PI3K) inhibitors wortmannin (100 nM) and LY294002 (20 µM). Testosterone (10 nM) had no effect on the doxorubicin-induced Cl(-) current. CONCLUSIONS AND IMPLICATIONS 17β-Oestradiol prevents the doxorubicin-induced cell shrinkage mediated through activation of I(Cl,vol) and subsequent induction of apoptosis signals, through a membrane receptor-dependent PI3K pathway in rabbit articular chondrocytes.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures



Similar articles
-
The COX-2 selective blocker etodolac inhibits TNFα-induced apoptosis in isolated rabbit articular chondrocytes.Int J Mol Sci. 2013 Sep 30;14(10):19705-15. doi: 10.3390/ijms141019705. Int J Mol Sci. 2013. PMID: 24084720 Free PMC article.
-
Regulatory role of tyrosine phosphorylation in the swelling-activated chloride current in isolated rabbit articular chondrocytes.J Physiol. 2009 Aug 1;587(Pt 15):3761-76. doi: 10.1113/jphysiol.2009.174177. Epub 2009 Jun 15. J Physiol. 2009. PMID: 19528252 Free PMC article.
-
Volume-sensitive chloride channels (ICl,vol) mediate doxorubicin-induced apoptosis through apoptotic volume decrease in cardiomyocytes.Fundam Clin Pharmacol. 2004 Oct;18(5):531-8. doi: 10.1111/j.1472-8206.2004.00273.x. Fundam Clin Pharmacol. 2004. PMID: 15482374
-
Activation of a chondrocyte volume-sensitive Cl(-) conductance prior to macroscopic cartilage lesion formation in the rabbit knee anterior cruciate ligament transection osteoarthritis model.Osteoarthritis Cartilage. 2016 Oct;24(10):1786-1794. doi: 10.1016/j.joca.2016.05.019. Epub 2016 Jun 4. Osteoarthritis Cartilage. 2016. PMID: 27266646 Free PMC article.
-
EGFR kinase regulates volume-sensitive chloride current elicited by integrin stretch via PI-3K and NADPH oxidase in ventricular myocytes.J Gen Physiol. 2006 Mar;127(3):237-51. doi: 10.1085/jgp.200509366. Epub 2006 Feb 13. J Gen Physiol. 2006. PMID: 16505146 Free PMC article.
Cited by
-
Activation of volume-sensitive Cl- channel mediates autophagy-related cell death in myocardial ischaemia/reperfusion injury.Oncotarget. 2016 Jun 28;7(26):39345-39362. doi: 10.18632/oncotarget.10050. Oncotarget. 2016. PMID: 27322431 Free PMC article.
-
The COX-2 selective blocker etodolac inhibits TNFα-induced apoptosis in isolated rabbit articular chondrocytes.Int J Mol Sci. 2013 Sep 30;14(10):19705-15. doi: 10.3390/ijms141019705. Int J Mol Sci. 2013. PMID: 24084720 Free PMC article.
-
Estradiol Inhibits ER Stress-Induced Apoptosis in Chondrocytes and Contributes to a Reduced Osteoarthritic Cartilage Degeneration in Female Mice.Front Cell Dev Biol. 2022 May 20;10:913118. doi: 10.3389/fcell.2022.913118. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35669511 Free PMC article.
-
Astrocytes Prevent Ethanol Induced Apoptosis of Nrf2 Depleted Neurons by Maintaining GSH Homeostasis.Open J Apoptosis. 2012 Jul;1(2):10.4236/ojapo.2012.12002. doi: 10.4236/ojapo.2012.12002. Open J Apoptosis. 2012. PMID: 24380057 Free PMC article.
-
Physiology of the volume-sensitive/regulatory anion channel VSOR/VRAC: part 2: its activation mechanisms and essential roles in organic signal release.J Physiol Sci. 2024 Jun 14;74(1):34. doi: 10.1186/s12576-024-00926-3. J Physiol Sci. 2024. PMID: 38877402 Free PMC article. Review.
References
-
- Adams CS, Shapiro IM. The fate of the terminally differentiated chondrocyte: evidence for microenvironmental regulation of chondrocyte apoptosis. Crit Rev Oral Biol Med. 2002;13:465–473. - PubMed
-
- d'Anglemont de Tassigny A, Souktani R, Henry P, Ghaleh B, Berdeaux A. Volume-sensitive chloride channels (ICl,vol) mediate doxorubicin-induced apoptosis through apoptotic volume decrease in cardiomyocytes. Fundam Clin Pharmacol. 2004;18:531–538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

