Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo
- PMID: 22142035
- PMCID: PMC3258504
- DOI: 10.1111/j.1365-2958.2011.07942.x
Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo
Abstract
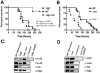
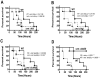
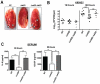
Bloodstream infection with Staphylococcus aureus is common and can be fatal. However, virulence factors that contribute to lethality in S. aureus bloodstream infection are poorly defined. We discovered that LukED, a commonly overlooked leucotoxin, is critical for S. aureus bloodstream infection in mice. We also determined that LukED promotes S. aureus replication in vivo by directly killing phagocytes recruited to sites of haematogenously seeded tissue. Furthermore, we established that murine neutrophils are the primary target of LukED, as the greater virulence of wild-type S. aureus compared with a lukED mutant was abrogated by depleting neutrophils. The in vivo toxicity of LukED towards murine phagocytes is unique among S. aureus leucotoxins, implying its crucial role in pathogenesis. Moreover, the tropism of LukED for murine phagocytes highlights the utility of murine models to study LukED pathobiology, including development and testing of strategies to inhibit toxin activity and control bacterial infection.
© 2011 Blackwell Publishing Ltd.
Figures

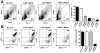


References
-
- Methicillin-resistant Staphylococcus aureus infections in correctional facilities---Georgia. Vol. 52. MMWR Morb Mortal Wkly Rep; California, and Texas: 2003. pp. 992–996. 2001-2003. - PubMed
-
- Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

