Effects of acutely inhibiting PI3K isoforms and mTOR on regulation of glucose metabolism in vivo
- PMID: 22142257
- PMCID: PMC3343648
- DOI: 10.1042/BJ20111913
Effects of acutely inhibiting PI3K isoforms and mTOR on regulation of glucose metabolism in vivo
Abstract
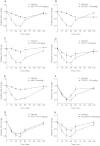
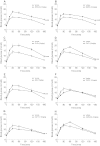
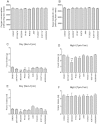
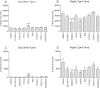
In in vitro studies class-I PI3Ks (phosphoinositide 3-kinases), class-II PI3Ks and mTOR (mammalian target of rapamycin) have all been described as having roles in the regulation of glucose metabolism. The relative role each plays in the normal signalling processes regulating glucose metabolism in vivo is less clear. Knockout and knockin mouse models have provided some evidence that the class-I PI3K isoforms p110α, p110β, and to a lesser extent p110γ, are necessary for processes regulating glucose metabolism and appetite. However, in these models the PI3K activity is chronically reduced. Therefore we analysed the effects of acutely inhibiting PI3K isoforms alone, or PI3K and mTOR, on glucose metabolism and food intake. In the present study impairments in glucose tolerance, insulin tolerance and increased hepatic glucose output were observed in mice treated with the pan-PI3K/mTOR inhibitors PI-103 and NVP-BEZ235. The finding that ZSTK474 has similar effects indicates that these effects are due to inhibition of PI3K rather than mTOR. The p110α-selective inhibitors PIK75 and A66 also induced these phenotypes, but inhibitors of p110β, p110δ or p110γ induced only minor effects. These drugs caused no significant effects on BMR (basal metabolic rate), O2 consumption or water intake, but BEZ235, PI-103 and PIK75 did cause a small reduction in food consumption. Surprisingly, pan-PI3K inhibitors or p110α inhibitors caused reductions in animal movement, although the cause of this is not clear. Taken together these studies provide pharmacological evidence to support a pre-eminent role for the p110α isoform of PI3K in pathways acutely regulating glucose metabolism.
Figures


Similar articles
-
Extended treatment with selective phosphatidylinositol 3-kinase and mTOR inhibitors has effects on metabolism, growth, behaviour and bone strength.FEBS J. 2013 Nov;280(21):5337-49. doi: 10.1111/febs.12428. Epub 2013 Aug 12. FEBS J. 2013. PMID: 23837532
-
Diverse mechanisms activate the PI 3-kinase/mTOR pathway in melanomas: implications for the use of PI 3-kinase inhibitors to overcome resistance to inhibitors of BRAF and MEK.BMC Cancer. 2021 Feb 6;21(1):136. doi: 10.1186/s12885-021-07826-4. BMC Cancer. 2021. PMID: 33549048 Free PMC article.
-
Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells.Cancer Biol Ther. 2011 Jun 1;11(11):938-46. doi: 10.4161/cbt.11.11.15527. Epub 2011 Jun 1. Cancer Biol Ther. 2011. PMID: 21464613 Free PMC article.
-
Drugging the PI3 kinome: from chemical tools to drugs in the clinic.Cancer Res. 2010 Mar 15;70(6):2146-57. doi: 10.1158/0008-5472.CAN-09-4355. Epub 2010 Feb 23. Cancer Res. 2010. PMID: 20179189 Free PMC article. Review.
-
Development of PI3K inhibitors: Advances in clinical trials and new strategies (Review).Pharmacol Res. 2021 Nov;173:105900. doi: 10.1016/j.phrs.2021.105900. Epub 2021 Sep 20. Pharmacol Res. 2021. PMID: 34547385 Review.
Cited by
-
Distinct and opposing roles for the phosphatidylinositol 3-OH kinase catalytic subunits p110α and p110β in the regulation of insulin secretion from rodent and human beta cells.Diabetologia. 2013 Jun;56(6):1339-49. doi: 10.1007/s00125-013-2882-4. Epub 2013 Apr 9. Diabetologia. 2013. PMID: 23568272
-
Efficacy of Providing the PI3K p110α Inhibitor BYL719 (Alpelisib) to Middle-Aged Mice in Their Diet.Biomolecules. 2021 Jan 25;11(2):150. doi: 10.3390/biom11020150. Biomolecules. 2021. PMID: 33503847 Free PMC article.
-
Modeling integrated cellular machinery using hybrid Petri-Boolean networks.PLoS Comput Biol. 2013;9(11):e1003306. doi: 10.1371/journal.pcbi.1003306. Epub 2013 Nov 7. PLoS Comput Biol. 2013. PMID: 24244124 Free PMC article.
-
For Better or Worse: The Potential for Dose Limiting the On-Target Toxicity of PI 3-Kinase Inhibitors.Biomolecules. 2019 Aug 22;9(9):402. doi: 10.3390/biom9090402. Biomolecules. 2019. PMID: 31443495 Free PMC article. Review.
-
Global expression profiling and pathway analysis of mouse mammary tumor reveals strain and stage specific dysregulated pathways in breast cancer progression.Cell Cycle. 2018;17(8):963-973. doi: 10.1080/15384101.2018.1442629. Epub 2018 May 31. Cell Cycle. 2018. PMID: 29712537 Free PMC article.
References
-
- Marone R., Cmiljanovic V., Giese B., Wymann M. P. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim. Biophys. Acta. 2008;1784:159–185. - PubMed
-
- Falasca M., Hughes W. E., Dominguez V., Sala G., Fostira F., Fang M. Q., Cazzolli R., Shepherd P. R., James D. E., Maffucci T. The role of phosphoinositide 3-kinase C2α in insulin signaling. J. Biol. Chem. 2007;282:28226–28236. - PubMed
-
- Brown R. A., Domin J., Arcaro A., Waterfield M. D., Shepherd P. R. Insulin activates the α-isoform of class II phosphoinositide 3-kinase. J. Biol. Chem. 1999;274:14529–14532. - PubMed
-
- Byfield M. P., Murray J. T., Backer J. M. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J. Biol. Chem. 2005;280:33076–33082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

