Rationale, design, and implementation protocol of the Dutch clinical practice guideline pain in patients with cancer: a cluster randomised controlled trial with Short Message Service (SMS) and Interactive Voice Response (IVR)
- PMID: 22142327
- PMCID: PMC3248867
- DOI: 10.1186/1748-5908-6-126
Rationale, design, and implementation protocol of the Dutch clinical practice guideline pain in patients with cancer: a cluster randomised controlled trial with Short Message Service (SMS) and Interactive Voice Response (IVR)
Abstract
Background: One-half of patients with cancer have pain. In nearly one out of two cancer patients with pain, this was undertreated. Inadequate pain control still remains an important problem in this group of patients. Therefore, in 2008 a national, evidence-based multidisciplinary clinical practice guideline 'pain in patients with cancer' has been developed. Yet, publishing a guideline is not enough. Implementation is needed to improve pain management. An innovative implementation strategy, Short Message Service with Interactive Voice Response (SVS-IVR), has been developed and pilot tested. This study aims to evaluate on effectiveness of this strategy to improve pain reporting, pain measurement and adequate pain therapy. In addition, whether the active role of the patient and involvement of caregivers in pain management may change.
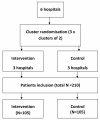
Methods/design: A cluster randomised controlled trial with two arms will be performed in six oncology outpatient clinics of hospitals in the Southeastern region of the Netherlands, with three hospitals in the intervention and three in the control condition. Follow-up measurements will be conducted in all hospitals to study the long-term effect of the intervention. The intervention includes training of professionals (medical oncologists, nurses, and general practitioners) and SMS-IVR to report pain in patients with cancer to improve pain reporting by patients, pain management by medical oncologists, nurses, and general practitioners, and decrease pain intensity.
Discussion: This innovative implementation strategy with technical tools and the involvement of patients, may enhance the use of the guideline 'pain in patients with cancer' for pain management. Short Message Service alerts may serve as a tool to support self-management of patients. Therefore, the SMS-IVR intervention may increase the feeling of having control over one's life.
Trial registration: [corrected] Netherlands Trial Register (NTR): NTR2739.
© 2011 te Boveldt et al; licensee BioMed Central Ltd.
Figures
References
-
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van KM, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. AnnOncol. 2007;18:1437–1449. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

