DLK1, delta-like 1 homolog (Drosophila), regulates tumor cell differentiation in vivo
- PMID: 22142700
- PMCID: PMC3243111
- DOI: 10.1016/j.canlet.2011.11.032
DLK1, delta-like 1 homolog (Drosophila), regulates tumor cell differentiation in vivo
Abstract
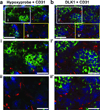
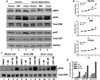
The stem cell-like characteristics of tumor cells are not only essential for tumor development and malignant progression, but also significantly contribute to therapy resistance. However, it remains poorly understood how cancer cell differentiation or stemness is regulated in vivo. We investigated the role of the stem cell gene DLK1, or delta-like 1 homolog (Drosophila), in the regulation of cancer cell differentiation in vivo using neuroblastoma (NB) xenografts as a model. We found that loss-of-function mutants of DLK1 significantly enhanced NB cell differentiation in vivo likely by increasing the basal phosphorylation of MEK and ERK kinases, a mechanism that has been shown to facilitate neuronal differentiation. We also found that DLK1(+) cells are preferentially located in hypoxic regions. These results clearly demonstrate that DLK1 plays an important role in the maintenance of undifferentiated, stem cell-like phenotypes of NB cells in vivo.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
None declared.
Figures




Similar articles
-
Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity.Cancer Res. 2009 Dec 15;69(24):9271-80. doi: 10.1158/0008-5472.CAN-09-1605. Cancer Res. 2009. PMID: 19934310 Free PMC article.
-
Effect of β-carotene on cancer cell stemness and differentiation in SK-N-BE(2)C neuroblastoma cells.Oncol Rep. 2013 Oct;30(4):1869-77. doi: 10.3892/or.2013.2643. Epub 2013 Jul 30. Oncol Rep. 2013. PMID: 23900747
-
Interaction of delta-like 1 homolog (Drosophila) with prohibitins and its impact on tumor cell clonogenicity.Mol Cancer Res. 2014 Jan;12(1):155-64. doi: 10.1158/1541-7786.MCR-13-0360. Epub 2013 Nov 18. Mol Cancer Res. 2014. PMID: 24249679 Free PMC article.
-
The imprinted gene Delta like non-canonical Notch ligand 1 (Dlk1) is conserved in mammals, and serves a growth modulatory role during tissue development and regeneration through Notch dependent and independent mechanisms.Cytokine Growth Factor Rev. 2019 Apr;46:17-27. doi: 10.1016/j.cytogfr.2019.03.006. Epub 2019 Mar 23. Cytokine Growth Factor Rev. 2019. PMID: 30930082 Review.
-
Emerging Roles of DLK1 in the Stem Cell Niche and Cancer Stemness.J Histochem Cytochem. 2022 Jan;70(1):17-28. doi: 10.1369/00221554211048951. Epub 2021 Oct 4. J Histochem Cytochem. 2022. PMID: 34606325 Free PMC article. Review.
Cited by
-
A proteogenomic surfaceome study identifies DLK1 as an immunotherapeutic target in neuroblastoma.Cancer Cell. 2024 Nov 11;42(11):1970-1982.e7. doi: 10.1016/j.ccell.2024.10.003. Epub 2024 Oct 24. Cancer Cell. 2024. PMID: 39454577
-
Actin cytoskeleton regulator Arp2/3 complex is required for DLL1 activating Notch1 signaling to maintain the stem cell phenotype of glioma initiating cells.Oncotarget. 2017 May 16;8(20):33353-33364. doi: 10.18632/oncotarget.16495. Oncotarget. 2017. PMID: 28380416 Free PMC article.
-
Anti-cancer stemness and anti-invasive activity of bitter taste receptors, TAS2R8 and TAS2R10, in human neuroblastoma cells.PLoS One. 2017 May 3;12(5):e0176851. doi: 10.1371/journal.pone.0176851. eCollection 2017. PLoS One. 2017. PMID: 28467517 Free PMC article.
-
Silencing YTHDF2 Induces Apoptosis of Neuroblastoma Cells In a Cell Line-Dependent Manner via Regulating the Expression of DLK1.Mol Neurobiol. 2025 Jul;62(7):8121-8134. doi: 10.1007/s12035-025-04759-y. Epub 2025 Feb 20. Mol Neurobiol. 2025. PMID: 39979690
-
Truncated DNMT3B isoform DNMT3B7 suppresses growth, induces differentiation, and alters DNA methylation in human neuroblastoma.Cancer Res. 2012 Sep 15;72(18):4714-23. doi: 10.1158/0008-5472.CAN-12-0886. Epub 2012 Jul 18. Cancer Res. 2012. PMID: 22815530 Free PMC article.
References
-
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. - PubMed
-
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. - PubMed
-
- Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118:409–418. - PubMed
-
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

