Successful disease-specific induced pluripotent stem cell generation from patients with kidney transplantation
- PMID: 22142803
- PMCID: PMC3340557
- DOI: 10.1186/scrt89
Successful disease-specific induced pluripotent stem cell generation from patients with kidney transplantation
Abstract
Introduction: End-stage renal disease (ESRD) is a major public health problem. Although kidney transplantation is a viable therapeutic option, this therapy is associated with significant limitations, including a shortage of donor organs. Induced pluripotent stem (iPS) cell technology, which allows derivation of patient-specific pluripotent stem cells, could provide a possible alternative modality for kidney replacement therapy for patients with ESRD.
Methods: The feasibility of iPS cell generation from patients with a history of ESRD was investigated using lentiviral vectors expressing pluripotency-associated factors.
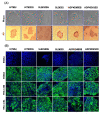
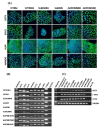
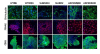
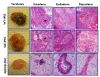
Results: In the present article we report, for the first time, generation of iPS cells from kidney transplant recipients with a history of autosomal-dominant polycystic kidney disease (ADPKD), systemic lupus erythematosus, or Wilms tumor and ESRD. Lentiviral transduction of OCT4, SOX2, KLF4 and c-MYC, under feeder-free conditions, resulted in reprogramming of skin-derived keratinocytes. Keratinocyte-derived iPS cells exhibited properties of human embryonic stem cells, including morphology, growth properties, expression of pluripotency genes and surface markers, spontaneous differentiation and teratoma formation. All iPS cell clones from the ADPKD patient retained the conserved W3842X mutation in exon 41 of the PKD1 gene.
Conclusions: Our results demonstrate successful iPS cell generation from patients with a history of ESRD, PKD1 gene mutation, or chronic immunosuppression. iPS cells from autosomal kidney diseases, such as ADPKD, would provide unique opportunities to study patient-specific disease pathogenesis in vitro.
Figures
Similar articles
-
Successful Derivation of an Induced Pluripotent Stem Cell Line from a Genetically Nonpermissive Enhanced Green Fluorescent Protein-Transgenic FVB/N Mouse Strain.Cell Reprogram. 2019 Oct;21(5):270-284. doi: 10.1089/cell.2019.0019. Cell Reprogram. 2019. PMID: 31596624
-
Generation of Mouse-Induced Pluripotent Stem Cells by Lentiviral Transduction.Methods Mol Biol. 2019;1940:63-76. doi: 10.1007/978-1-4939-9086-3_5. Methods Mol Biol. 2019. PMID: 30788818
-
Efficient generation of nonhuman primate induced pluripotent stem cells.Stem Cells Dev. 2011 May;20(5):795-807. doi: 10.1089/scd.2010.0343. Epub 2011 Feb 1. Stem Cells Dev. 2011. PMID: 21058905 Free PMC article.
-
[Induction and characterization of induced pluripotent stem (iPS) cells: a review].Sheng Wu Gong Cheng Xue Bao. 2010 Apr;26(4):421-30. Sheng Wu Gong Cheng Xue Bao. 2010. PMID: 20575428 Review. Chinese.
-
Induced pluripotent stem cells from swine (Sus scrofa): why they may prove to be important.Cell Cycle. 2009 Oct 1;8(19):3078-81. doi: 10.4161/cc.8.19.9589. Epub 2009 Oct 21. Cell Cycle. 2009. PMID: 19738434 Review.
Cited by
-
Generation of systemic lupus erythematosus-specific induced pluripotent stem cells from urine.Rheumatol Int. 2013 Aug;33(8):2127-34. doi: 10.1007/s00296-013-2704-5. Epub 2013 Feb 22. Rheumatol Int. 2013. PMID: 23430158
-
Generation of disease-specific induced pluripotent stem cells from patients with rheumatoid arthritis and osteoarthritis.Arthritis Res Ther. 2014 Feb 4;16(1):R41. doi: 10.1186/ar4470. Arthritis Res Ther. 2014. PMID: 24490617 Free PMC article.
-
Regenerative Medicine, Disease Modeling, and Drug Discovery in Human Pluripotent Stem Cell-derived Kidney Tissue.Eur Med J Reprod Health. 2017 Aug;3(1):57-67. Eur Med J Reprod Health. 2017. PMID: 31157117 Free PMC article.
-
An Overview of In Vivo and In Vitro Models for Autosomal Dominant Polycystic Kidney Disease: A Journey from 3D-Cysts to Mini-Pigs.Int J Mol Sci. 2020 Jun 25;21(12):4537. doi: 10.3390/ijms21124537. Int J Mol Sci. 2020. PMID: 32630605 Free PMC article. Review.
-
Tissue Engineering and Stem Cell Therapy in Pediatric Urology.J Indian Assoc Pediatr Surg. 2019 Oct-Dec;24(4):237-246. doi: 10.4103/jiaps.JIAPS_77_18. J Indian Assoc Pediatr Surg. 2019. PMID: 31571753 Free PMC article. Review.
References
-
- Daar AS. The case for a regulated system of living kidney sales. Nat Clin Pract Nephrol. 2006;2:600–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

