Environmental exposure, estrogen and two X chromosomes are required for disease development in an epigenetic model of lupus
- PMID: 22142890
- PMCID: PMC3312994
- DOI: 10.1016/j.jaut.2011.11.001
Environmental exposure, estrogen and two X chromosomes are required for disease development in an epigenetic model of lupus
Abstract
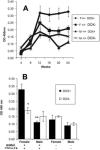
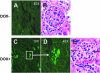
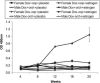
Systemic lupus erythematosus (SLE) is an autoimmune disease primarily afflicting women. The reason for the gender bias is unclear, but genetic susceptibility, estrogen and environmental agents appear to play significant roles in SLE pathogenesis. Environmental agents can contribute to lupus susceptibility through epigenetic mechanisms. We used (C57BL/6xSJL)F1 mice transgenic for a dominant-negative MEK (dnMEK) that was previously shown to be inducibly and selectively expressed in T cells. In this model, induction of the dnMEK by doxycycline treatment suppresses T cell ERK signaling, decreasing DNA-methyltransferase expression and resulting in DNA demethylation, overexpression of immune genes Itgal (CD11a) and Tnfsf7 (CD70), and anti-dsDNA antibody. To examine the role of gender and estrogen in this model, male and female transgenic mice were neutered and implanted with time-release pellets delivering placebo or estrogen. Doxycycline induced IgG anti-dsDNA antibodies in intact and neutered, placebo-treated control female but not male transgenic mice. Glomerular IgG deposits were also found in the kidneys of female but not male transgenic mice, and not in the absence of doxycycline. Estrogen enhanced anti-dsDNA IgG antibodies only in transgenic, ERK-impaired female mice. Decreased ERK activation also resulted in overexpression and demethylation of the X-linked methylation-sensitive gene CD40lg in female but not male mice, consistent with demethylation of the second X chromosome in the females. The results show that both estrogen and female gender contribute to the female predisposition in lupus susceptibility through hormonal and epigenetic X-chromosome effects and through suppression of ERK signaling by environmental agents.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
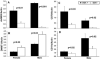

Similar articles
-
CD4(+) T cells epigenetically modified by oxidative stress cause lupus-like autoimmunity in mice.J Autoimmun. 2015 Aug;62:75-80. doi: 10.1016/j.jaut.2015.06.004. Epub 2015 Jul 9. J Autoimmun. 2015. PMID: 26165613 Free PMC article.
-
Diet influences expression of autoimmune-associated genes and disease severity by epigenetic mechanisms in a transgenic mouse model of lupus.Arthritis Rheum. 2013 Jul;65(7):1872-81. doi: 10.1002/art.37967. Arthritis Rheum. 2013. PMID: 23576011 Free PMC article.
-
Defective T-cell ERK signaling induces interferon-regulated gene expression and overexpression of methylation-sensitive genes similar to lupus patients.Genes Immun. 2008 Jun;9(4):368-78. doi: 10.1038/gene.2008.29. Genes Immun. 2008. PMID: 18523434 Free PMC article.
-
Epigenetic regulation and the pathogenesis of systemic lupus erythematosus.Transl Res. 2009 Jan;153(1):4-10. doi: 10.1016/j.trsl.2008.10.007. Epub 2008 Nov 14. Transl Res. 2009. PMID: 19100952 Review.
-
Impaired DNA methylation and its mechanisms in CD4(+)T cells of systemic lupus erythematosus.J Autoimmun. 2013 Mar;41:92-9. doi: 10.1016/j.jaut.2013.01.005. Epub 2013 Jan 20. J Autoimmun. 2013. PMID: 23340289 Review.
Cited by
-
T cell PKCδ kinase inactivation induces lupus-like autoimmunity in mice.Clin Immunol. 2015 Jun;158(2):193-203. doi: 10.1016/j.clim.2015.03.017. Epub 2015 Mar 28. Clin Immunol. 2015. PMID: 25829232 Free PMC article.
-
Environmental exposures and the development of systemic lupus erythematosus.Curr Opin Rheumatol. 2016 Sep;28(5):497-505. doi: 10.1097/BOR.0000000000000318. Curr Opin Rheumatol. 2016. PMID: 27428889 Free PMC article. Review.
-
Worldwide Incidence and Prevalence of Neuromyelitis Optica: A Systematic Review.Neurology. 2021 Jan 12;96(2):59-77. doi: 10.1212/WNL.0000000000011153. Epub 2020 Dec 11. Neurology. 2021. PMID: 33310876 Free PMC article.
-
Human papillomavirus vaccine and systemic lupus erythematosus.Clin Rheumatol. 2013 Sep;32(9):1301-7. doi: 10.1007/s10067-013-2266-7. Epub 2013 Apr 28. Clin Rheumatol. 2013. PMID: 23624585
-
CD4(+) T cells epigenetically modified by oxidative stress cause lupus-like autoimmunity in mice.J Autoimmun. 2015 Aug;62:75-80. doi: 10.1016/j.jaut.2015.06.004. Epub 2015 Jul 9. J Autoimmun. 2015. PMID: 26165613 Free PMC article.
References
-
- S.L.E. Lupus Foundation Website. New York, NY: http://www.lupusny.org/about-lupus/who-gets-lupus.
-
- Pan Y, Sawalha AH. Epigenetic regulation and the pathogenesis of systemic lupus erythematosus. Transl Res. 2009;153:4–10. - PubMed
-
- Kelly JA, Moser KL, Harley JB. The genetics of systemic lupus erythematosus: putting the pieces together. Genes Immun. 2002;3(Suppl 1):S71–85. - PubMed
-
- Castro J, Balada E, Ordi-Ros J, Vilardell-Tarrés M. The complex immunogenetic basis of systemic lupus erythematosus. Autoimmunity Reviews. 2008;7:345–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG020628/AG/NIA NIH HHS/United States
- ES015214/ES/NIEHS NIH HHS/United States
- R01AG020628/AG/NIA NIH HHS/United States
- P30 AG024824/AG/NIA NIH HHS/United States
- R01AG028268/AG/NIA NIH HHS/United States
- P30 ES017885/ES/NIEHS NIH HHS/United States
- P30 AG013283/AG/NIA NIH HHS/United States
- R01 AG028268/AG/NIA NIH HHS/United States
- R01 ES015214/ES/NIEHS NIH HHS/United States
- R01 AR042525/AR/NIAMS NIH HHS/United States
- AG013283/AG/NIA NIH HHS/United States
- P30ES017885/ES/NIEHS NIH HHS/United States
- AR42525/AR/NIAMS NIH HHS/United States
- P30AG024824/AG/NIA NIH HHS/United States
- R01AR042525/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

