Effect of progestin compared with combined oral contraceptive pills on lactation: a randomized controlled trial
- PMID: 22143258
- PMCID: PMC3586805
- DOI: 10.1097/AOG.0b013e31823dc015
Effect of progestin compared with combined oral contraceptive pills on lactation: a randomized controlled trial
Abstract
Objective: To estimate the effect of progestin-only compared with combined hormonal contraceptive pills on rates of breastfeeding continuation in postpartum women. Secondary outcomes include infant growth parameters, contraceptive method continuation, and patient satisfaction with breastfeeding and contraceptive method.
Methods: Postpartum breastfeeding women who desired oral contraceptives were randomly assigned to progestin-only and combined hormonal contraceptive pills. At 2 and 8 weeks postpartum, participants completed in-person questionnaires that assessed breastfeeding continuation and contraceptive use. Infant growth parameters including weight, length, and head circumference were assessed at 8 weeks postpartum. Telephone questionnaires assessing breastfeeding, contraceptive continuation, and satisfaction were completed at 3-7 weeks and 4 and 6 months. Breastfeeding continuation was compared between groups using Cox proportional hazards regression. Differences in baseline demographic characteristics and in variables between the two intervention groups were compared using χ tests, Fisher exact test, or two-sample t tests as appropriate.
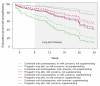
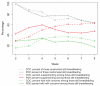
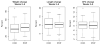
Results: Breastfeeding continuation rates at 8 weeks (progestin-only 63.5%; combined hormonal 64.1%), contraceptive continuation, and infant growth parameters did not differ between users of progestin-only and combined hormonal contraceptive pills. Infant formula supplementation and maternal perception of inadequate milk supply were associated with decreased rates of breastfeeding in both groups.
Conclusion: Choice of combined hormonal or progestin-only contraceptive pills administered 2 weeks postpartum did not adversely affect breastfeeding continuation.
Clinical trial registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT01465022.
Figures
Comment in
-
Exploring contraceptive options for breastfeeding mothers.Obstet Gynecol. 2012 Jan;119(1):1-2. doi: 10.1097/AOG.0b013e31823f3c6d. Obstet Gynecol. 2012. PMID: 22183205 No abstract available.
References
-
- Horta B, Bahl R, Martines J, Victora C. Evidence of the long-term effects of breastfeeding; systematic review and meta-analysis. World Health Organization; Geneva, Switzerland: 2007.
-
- Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. - PubMed
-
- Miller GH, Hughes LR. Lactation and genital involution effects of a new low-dose oral contraceptive on breast-feeding mothers and their infants. Obstet Gynecol. 1970 Jan;35(1):44–50. - PubMed
-
- Diaz S, Peralta O, Juez G, Herreros C, Casado ME, Salvatierra AM, et al. Fertility regulation in nursing women: III. Short-term influence of a low-dose combined oral contraceptive upon lactation and infant growth. Contraception. 1983;27(1):1–11. - PubMed
-
- Croxatto HB, Diaz S, Peralta O, Juez G, Herreros C, Casado ME, et al. Fertility regulation in nursing women: IV. Long-term influence of a low-dose combined oral contraceptive initiated at day 30 postpartum upon lactation and infant growth. Contraception. 1983;27(1):13–25. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

