Genetic background of Prop1(df) mutants provides remarkable protection against hypothyroidism-induced hearing impairment
- PMID: 22143287
- PMCID: PMC3298611
- DOI: 10.1007/s10162-011-0302-3
Genetic background of Prop1(df) mutants provides remarkable protection against hypothyroidism-induced hearing impairment
Abstract
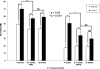
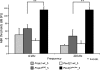
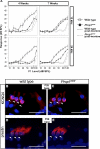
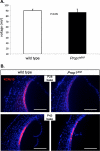
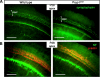
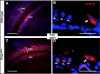
Hypothyroidism is a cause of genetic and environmentally induced deafness. The sensitivity of cochlear development and function to thyroid hormone (TH) mandates understanding TH action in this sensory organ. Prop1(df) and Pou1f1(dw) mutant mice carry mutations in different pituitary transcription factors, each resulting in pituitary thyrotropin deficiency. Despite the same lack of detectable serum TH, these mutants have very different hearing abilities: Prop1(df) mutants are mildly affected, while Pou1f1(dw) mutants are completely deaf. Genetic studies show that this difference is attributable to the genetic backgrounds. Using embryo transfer, we discovered that factors intrinsic to the fetus are the major contributor to this difference, not maternal effects. We analyzed Prop1(df) mutants to identify processes in cochlear development that are disrupted in other hypothyroid animal models but protected in Prop1(df) mutants by the genetic background. The development of outer hair cell (OHC) function is delayed, but Prestin and KCNQ4 immunostaining appear normal in mature Prop1(df) mutants. The endocochlear potential and KCNJ10 immunostaining in the stria vascularis are indistinguishable from wild type, and no differences in neurofilament or synaptophysin staining are evident in Prop1(df) mutants. The synaptic vesicle protein otoferlin normally shifts expression from OHC to IHC as temporary afferent fibers beneath the OHC regress postnatally. Prop1(df) mutants exhibit persistent, abnormal expression of otoferlin in apical OHC, suggesting delayed maturation of synaptic function. Thus, the genetic background of Prop1(df) mutants is remarkably protective for most functions affected in other hypothyroid mice. The Prop1(df) mutant is an attractive model for identifying the genes that protect against deafness.
Figures
Similar articles
-
Genetic variation in thyroid folliculogenesis influences susceptibility to hypothyroidism-induced hearing impairment.Mamm Genome. 2019 Feb;30(1-2):5-22. doi: 10.1007/s00335-019-09792-6. Epub 2019 Feb 18. Mamm Genome. 2019. PMID: 30778664 Free PMC article.
-
Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants.J Neurosci. 2009 Jan 28;29(4):1212-23. doi: 10.1523/JNEUROSCI.4957-08.2009. J Neurosci. 2009. PMID: 19176829 Free PMC article.
-
Candidate genes for panhypopituitarism identified by gene expression profiling.Physiol Genomics. 2011 Oct 6;43(19):1105-16. doi: 10.1152/physiolgenomics.00080.2011. Epub 2011 Aug 9. Physiol Genomics. 2011. PMID: 21828248 Free PMC article.
-
Combined pituitary hormone deficiency: role of Pit-1 and Prop-1.Acta Paediatr Suppl. 1999 Dec;88(433):33-41. doi: 10.1111/j.1651-2227.1999.tb14401.x. Acta Paediatr Suppl. 1999. PMID: 10626543 Review.
-
Pituitary transcription factors in the aetiology of combined pituitary hormone deficiency.Best Pract Res Clin Endocrinol Metab. 2011 Feb;25(1):43-60. doi: 10.1016/j.beem.2010.10.014. Best Pract Res Clin Endocrinol Metab. 2011. PMID: 21396574 Review.
Cited by
-
Hearing impairment in hypothyroid dwarf mice caused by mutations of the thyroid peroxidase gene.J Assoc Res Otolaryngol. 2014 Feb;15(1):45-55. doi: 10.1007/s10162-013-0427-7. Epub 2013 Dec 3. J Assoc Res Otolaryngol. 2014. PMID: 24297261 Free PMC article.
-
Genetic variation in thyroid folliculogenesis influences susceptibility to hypothyroidism-induced hearing impairment.Mamm Genome. 2019 Feb;30(1-2):5-22. doi: 10.1007/s00335-019-09792-6. Epub 2019 Feb 18. Mamm Genome. 2019. PMID: 30778664 Free PMC article.
-
Novel Nucleotide Variations, Haplotypes Structure and Associations with Growth Related Traits of Goat AT Motif-Binding Factor (ATBF1) Gene.Asian-Australas J Anim Sci. 2015 Oct;28(10):1394-406. doi: 10.5713/ajas.14.0860. Asian-Australas J Anim Sci. 2015. PMID: 26323396 Free PMC article.
-
A lack of immune system genes causes loss in high frequency hearing but does not disrupt cochlear synapse maturation in mice.PLoS One. 2014 May 7;9(5):e94549. doi: 10.1371/journal.pone.0094549. eCollection 2014. PLoS One. 2014. PMID: 24804771 Free PMC article.
-
Atrophic thyroid follicles and inner ear defects reminiscent of cochlear hypothyroidism in Slc26a4-related deafness.Mamm Genome. 2014 Aug;25(7-8):304-16. doi: 10.1007/s00335-014-9515-1. Epub 2014 Apr 24. Mamm Genome. 2014. PMID: 24760582 Free PMC article.
References
-
- Brandt N, Kuhn S, Munkner S, Braig C, Winter H, Blin N, Vonthein R, Knipper M, Engel J. Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells. J Neurosci. 2007;27:3174–3186. doi: 10.1523/JNEUROSCI.3965-06.2007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC009590/DC/NIDCD NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- R01DC009590/DC/NIDCD NIH HHS/United States
- AR20557/AR/NIAMS NIH HHS/United States
- CA46592/CA/NCI NIH HHS/United States
- DK20572/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- P30 DC05188/DC/NIDCD NIH HHS/United States
- DK34933/DK/NIDDK NIH HHS/United States
- P30 DC005188/DC/NIDCD NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- P30DK08194/DK/NIDDK NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

