Ca(2+) channel currents of cortical neurons from pure and mixed cultures
- PMID: 22143344
- PMCID: PMC3279576
- DOI: 10.1007/s10616-011-9405-2
Ca(2+) channel currents of cortical neurons from pure and mixed cultures
Abstract
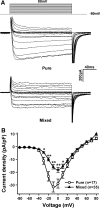
Voltage-gated Ca(2+) channels (VGCCs) are key regulators of many neuronal functions, and involved in multiple central nervous system diseases. In the last 30 years, a large number of injury and disease models have been established based on cultured neurons. Culture with serum develops a mixture of neurons and glial cells, while culture without serum develops pure neurons. Both of these neuronal-culture methods are widely used. However, the properties of Ca(2+) currents in neurons from these two cultures have not been compared. In this study, we cultured rat cortical neurons in serum-containing or -free medium and then recorded the Ca(2+) channel currents using patch-clamp technique. Our results showed that there were significant differences in the amplitude and activation properties of whole-cell Ca(2+) channel currents, and of non-L-type Ca(2+) channel currents between the neurons from these two culture systems. Our data suggested that the difference of whole-cell Ca(2+) currents may result from the differences in non-L-type currents. Understanding of these properties will considerably advance studies of VGCCs in neurons from pure or mixed culture.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

