Phase I study for ridaforolimus, an oral mTOR inhibitor, in Japanese patients with advanced solid tumors
- PMID: 22143378
- PMCID: PMC3313018
- DOI: 10.1007/s00280-011-1788-4
Phase I study for ridaforolimus, an oral mTOR inhibitor, in Japanese patients with advanced solid tumors
Abstract
Purpose: Ridaforolimus is a non-prodrug mTOR inhibitor. The safety, pharmacokinetics (PK), and antitumor activity of oral ridaforolimus were assessed in Japanese patients with refractory solid tumors.
Methods: Ridaforolimus (20 or 40 mg) was administered as a single dose on Day 1, followed by once daily dosing five times a week for a 3-week cycle beginning on Day 8. Full PK sampling was performed on Days 1 and 26.
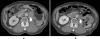
Results: Thirteen patients (7 at 20 mg and 6 at 40 mg) were enrolled. The median treatment duration was 82 days. The most common drug-related adverse events were stomatitis, hypertriglyceridemia, and proteinuria. Two patients had dose-limiting toxicities (grade 3 stomatitis at 20 mg, and grade 3 anorexia and vomiting at 40 mg). Four patients had grade 1 interstitial pneumonitis. Ridaforolimus in the whole blood was rapidly absorbed and slowly eliminated with a half-life of approximately 56-58 h after a single dose. Two patients (with non-small cell lung cancer and angiosarcoma, respectively) achieved a partial response, and five patients (one with thymic cancer and four with soft tissue sarcomas) had a stable disease for ≥ 16 weeks.
Conclusions: Ridaforolimus was well tolerated up to a dose of 40 mg in Japanese patients. Preliminary evidence of antitumor activity was observed for patients with solid tumors. Further investigation at this dose is warranted.
Figures
References
-
- Clackson T, Metcalf CA, Rivera VM, et al (2003) Broad anti-tumor activity of ap23573, an mTOR inhibitor in clinical development. Am Soc Clin Oncol (Meeting Proceedings) 22:220, abstr 882
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous