E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis
- PMID: 22143530
- PMCID: PMC3264227
- DOI: 10.1128/AAC.00731-11
E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis
Abstract
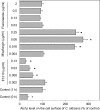
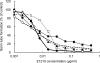
Continued research toward the development of new antifungals that act via inhibition of glycosylphosphatidylinositol (GPI) biosynthesis led to the design of E1210. In this study, we assessed the selectivity of the inhibitory activity of E1210 against Candida albicans GWT1 (Orf19.6884) protein, Aspergillus fumigatus GWT1 (AFUA_1G14870) protein, and human PIG-W protein, which can catalyze the inositol acylation of GPI early in the GPI biosynthesis pathway, and then we assessed the effects of E1210 on key C. albicans virulence factors. E1210 inhibited the inositol acylation activity of C. albicans Gwt1p and A. fumigatus Gwt1p with 50% inhibitory concentrations (IC(50)s) of 0.3 to 0.6 μM but had no inhibitory activity against human Pig-Wp even at concentrations as high as 100 μM. To confirm the inhibition of fungal GPI biosynthesis, expression of ALS1 protein, a GPI-anchored protein, on the surfaces of C. albicans cells treated with E1210 was studied and shown to be significantly lower than that on untreated cells. However, the ALS1 protein levels in the crude extract and the RHO1 protein levels on the cell surface were found to be almost the same. Furthermore, E1210 inhibited germ tube formation, adherence to polystyrene surfaces, and biofilm formation of C. albicans at concentrations above its MIC. These results suggested that E1210 selectively inhibited inositol acylation of fungus-specific GPI which would be catalyzed by Gwt1p, leading to the inhibition of GPI-anchored protein maturation, and also that E1210 suppressed the expression of some important virulence factors of C. albicans, through its GPI biosynthesis inhibition.
Figures










References
-
- Abe S, Satoh T, Tokuda Y, Tansho S, Yamaguchi H. 1994. A rapid colorimetric assay for determination of leukocyte-mediated inhibition of mycelial growth of Candida albicans. Microbiol. Immunol. 38: 385–388 - PubMed
-
- Albrecht A, et al. 2006. Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J. Biol. Chem. 281: 688–694 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

