Signal control through Raf: in sickness and in health
- PMID: 22143568
- PMCID: PMC3351917
- DOI: 10.1038/cr.2011.193
Signal control through Raf: in sickness and in health
Abstract
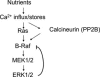
The extracellular signal-regulated kinase 1/2 (ERK1/2) cascade is the prototype mammalian mitogen-activated protein kinase (MAPK) signaling cascade that regulates a number of processes, including proliferation, differentiation, survival, migration, stress responses and apoptosis. How this seemingly linear cascade is modulated to achieve a specific cellular function has been a main focus of the field. In this review, we describe new as well as old findings in the regulation of the ERK1/2 pathway in normal and disease states via MAP3Ks.
Figures

Similar articles
-
RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth.Nature. 2010 Mar 18;464(7287):431-5. doi: 10.1038/nature08833. Epub 2010 Feb 3. Nature. 2010. PMID: 20130576
-
Calcineurin increases glucose activation of ERK1/2 by reversing negative feedback.Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22314-9. doi: 10.1073/pnas.1016630108. Epub 2010 Dec 6. Proc Natl Acad Sci U S A. 2010. PMID: 21135229 Free PMC article.
-
C-Raf inhibits MAPK activation and transformation by B-Raf(V600E).Mol Cell. 2009 Nov 13;36(3):477-86. doi: 10.1016/j.molcel.2009.10.017. Mol Cell. 2009. PMID: 19917255
-
RAF protein-serine/threonine kinases: structure and regulation.Biochem Biophys Res Commun. 2010 Aug 27;399(3):313-7. doi: 10.1016/j.bbrc.2010.07.092. Epub 2010 Jul 30. Biochem Biophys Res Commun. 2010. PMID: 20674547 Review.
-
Structural snapshots of RAF kinase interactions.Biochem Soc Trans. 2018 Dec 17;46(6):1393-1406. doi: 10.1042/BST20170528. Epub 2018 Oct 31. Biochem Soc Trans. 2018. PMID: 30381334 Review.
Cited by
-
The Upstream Pathway of mTOR-Mediated Autophagy in Liver Diseases.Cells. 2019 Dec 9;8(12):1597. doi: 10.3390/cells8121597. Cells. 2019. PMID: 31835352 Free PMC article. Review.
-
The role of ERK1/2 signaling in diabetes: pathogenic and therapeutic implications.Front Pharmacol. 2025 May 9;16:1600251. doi: 10.3389/fphar.2025.1600251. eCollection 2025. Front Pharmacol. 2025. PMID: 40417223 Free PMC article. Review.
-
The nuclear translocation of ERK1/2 as an anticancer target.Nat Commun. 2015 Mar 30;6:6685. doi: 10.1038/ncomms7685. Nat Commun. 2015. PMID: 25819065
-
Acute mitochondrial inhibition by mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) 1/2 inhibitors regulates proliferation.J Biol Chem. 2013 Feb 1;288(5):2933-40. doi: 10.1074/jbc.M112.430082. Epub 2012 Dec 12. J Biol Chem. 2013. PMID: 23235157 Free PMC article.
-
Muscle-derived extracellular signal-regulated kinases 1 and 2 are required for the maintenance of adult myofibers and their neuromuscular junctions.Mol Cell Biol. 2015 Apr;35(7):1238-53. doi: 10.1128/MCB.01071-14. Epub 2015 Jan 20. Mol Cell Biol. 2015. PMID: 25605336 Free PMC article.
References
-
- Boulton TG, Yancopoulos GD, Gregory JS, et al. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990;249:64–67. - PubMed
-
- Robinson LC, Gibbs JB, Marshall MS, Sigal IS, Tatchell K. CDC25: a component of the RAS-adenylate cyclase pathway in Saccharomyces cerevisiae. Science. 1987;235:1218–1221. - PubMed
-
- Broek D, Toda T, Michaeli T, et al. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987;48:789–799. - PubMed
-
- Simon MA, Bowtell DD, Dodson GS, Laverty TR, Rubin GM. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991;67:701–716. - PubMed
-
- Olivier JP, Raabe T, Henkemeyer M, et al. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange. Sos. Cell. 1993;73:179–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

