Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach
- PMID: 22143763
- PMCID: PMC3251151
- DOI: 10.1073/pnas.1111014108
Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach
Abstract
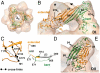
The molecular chaperone αB-crystallin, the major player in maintaining the transparency of the eye lens, prevents stress-damaged and aging lens proteins from aggregation. In nonlenticular cells, it is involved in various neurological diseases, diabetes, and cancer. Given its structural plasticity and dynamics, structure analysis of αB-crystallin presented hitherto a formidable challenge. Here we present a pseudoatomic model of a 24-meric αB-crystallin assembly obtained by a triple hybrid approach combining data from cryoelectron microscopy, NMR spectroscopy, and structural modeling. The model, confirmed by cross-linking and mass spectrometry, shows that the subunits interact within the oligomer in different, defined conformations. We further present the molecular architectures of additional well-defined αB-crystallin assemblies with larger or smaller numbers of subunits, provide the mechanism how "heterogeneity" is achieved by a small set of defined structural variations, and analyze the factors modulating the oligomer equilibrium of αB-crystallin and thus its chaperone activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

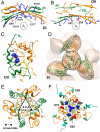
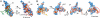

References
-
- Bloemendal H, et al. Ageing and vision: Structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. - PubMed
-
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. - PubMed
-
- Bloemendal H. The vertebrate eye lens. A useful system for the study of fundamental biological processes on a molecular level. Science. 1977;197:127–138. - PubMed
-
- de Jong WW, Caspers GJ, Leunissen JAM. Genealogy of the α-crystallin-small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

