Ouabain modulates ciliogenesis in epithelial cells
- PMID: 22143774
- PMCID: PMC3251114
- DOI: 10.1073/pnas.1102617108
Ouabain modulates ciliogenesis in epithelial cells
Abstract
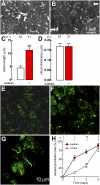
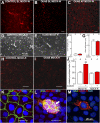
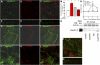
The exchange of substances between higher organisms and the environment occurs across transporting epithelia whose basic features are tight junctions (TJs) that seal the intercellular space, and polarity, which enables cells to transport substances vectorially. In a previous study, we demonstrated that 10 nM ouabain modulates TJs, and we now show that it controls polarity as well. We gauge polarity through the development of a cilium at the apical domain of Madin-Darby canine kidney cells (MDCK, epithelial dog kidney). Ouabain accelerates ciliogenesis in an ERK1/2-dependent manner. Claudin-2, a molecule responsible for the Na(+) and H(2)O permeability of the TJs, is also present at the cilium, as it colocalizes and coprecipitates with acetylated α-tubulin. Ouabain modulates claudin-2 localization at the cilium through ERK1/2. Comparing wild-type and ouabain-resistant MDCK cells, we show that ouabain acts through Na(+),K(+)-ATPase. Taken together, our previous and present results support the possibility that ouabain constitutes a hormone that modulates the transporting epithelial phenotype, thereby playing a crucial role in metazoan life.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The emergence of the concept of tight junctions and physiological regulation by ouabain.Semin Cell Dev Biol. 2014 Dec;36:149-56. doi: 10.1016/j.semcdb.2014.09.010. Epub 2014 Sep 19. Semin Cell Dev Biol. 2014. PMID: 25242280 Review.
-
Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells.Mol Biol Cell. 2001 Dec;12(12):3717-32. doi: 10.1091/mbc.12.12.3717. Mol Biol Cell. 2001. PMID: 11739775 Free PMC article.
-
Ouabain Modulates the Adherens Junction in Renal Epithelial Cells.Cell Physiol Biochem. 2019;52(6):1381-1397. doi: 10.33594/000000097. Cell Physiol Biochem. 2019. PMID: 31075189
-
The Na+-K+-ATPase as self-adhesion molecule and hormone receptor.Am J Physiol Cell Physiol. 2012 Feb 1;302(3):C473-81. doi: 10.1152/ajpcell.00083.2011. Epub 2011 Nov 2. Am J Physiol Cell Physiol. 2012. PMID: 22049208 Review.
-
Ouabain-induced alterations of the apical junctional complex involve α1 and β1 Na,K-ATPase downregulation and ERK1/2 activation independent of caveolae in colorectal cancer cells.J Membr Biol. 2014 Jan;247(1):23-33. doi: 10.1007/s00232-013-9607-y. Epub 2013 Nov 2. J Membr Biol. 2014. PMID: 24186357
Cited by
-
Claudin-2: Roles beyond Permeability Functions.Int J Mol Sci. 2019 Nov 12;20(22):5655. doi: 10.3390/ijms20225655. Int J Mol Sci. 2019. PMID: 31726679 Free PMC article. Review.
-
Ouabain promotes claudin-1, -2, and -4 autophagic degradation through oxidative stress and AMPK activation in MDCK cells.Autophagy Rep. 2023 Oct 2;2(1):2256146. doi: 10.1080/27694127.2023.2256146. eCollection 2023. Autophagy Rep. 2023. PMID: 40395300 Free PMC article.
-
Influence of Endogenous Cardiac Glycosides, Digoxin, and Marinobufagenin in the Physiology of Epithelial Cells.Cardiol Res Pract. 2019 Dec 30;2019:8646787. doi: 10.1155/2019/8646787. eCollection 2019. Cardiol Res Pract. 2019. PMID: 32089875 Free PMC article.
-
Primary cilia and kidney injury: current research status and future perspectives.Am J Physiol Renal Physiol. 2013 Oct 15;305(8):F1085-98. doi: 10.1152/ajprenal.00399.2013. Epub 2013 Jul 31. Am J Physiol Renal Physiol. 2013. PMID: 23904226 Free PMC article. Review.
-
Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes.J Cell Sci. 2012 Oct 15;125(Pt 20):4902-12. doi: 10.1242/jcs.111237. Epub 2012 Jul 23. J Cell Sci. 2012. PMID: 22825868 Free PMC article.
References
-
- Komiyama Y, et al. Identification of endogenous ouabain in culture supernatant of PC12 cells. J Hypertens. 2001;19:229–236. - PubMed
-
- Schneider R, et al. Bovine adrenals contain, in addition to ouabain, a second inhibitor of the sodium pump. J Biol Chem. 1998;273:784–792. - PubMed
-
- Bauer N, et al. Ouabain-like compound changes rapidly on physical exercise in humans and dogs: Effects of beta-blockade and angiotensin-converting enzyme inhibition. Hypertension. 2005;45:1024–1028. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

