A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge
- PMID: 22143779
- PMCID: PMC3251076
- DOI: 10.1073/pnas.1117715108
A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge
Abstract
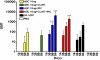
Ebola hemorrhagic fever is an acute and often deadly disease caused by Ebola virus (EBOV). The possible intentional use of this virus against human populations has led to design of vaccines that could be incorporated into a national stockpile for biological threat reduction. We have evaluated the immunogenicity and efficacy of an EBOV vaccine candidate in which the viral surface glycoprotein is biomanufactured as a fusion to a monoclonal antibody that recognizes an epitope in glycoprotein, resulting in the production of Ebola immune complexes (EICs). Although antigen-antibody immune complexes are known to be efficiently processed and presented to immune effector cells, we found that codelivery of the EIC with Toll-like receptor agonists elicited a more robust antibody response in mice than did EIC alone. Among the compounds tested, polyinosinic:polycytidylic acid (PIC, a Toll-like receptor 3 agonist) was highly effective as an adjuvant agent. After vaccinating mice with EIC plus PIC, 80% of the animals were protected against a lethal challenge with live EBOV (30,000 LD(50) of mouse adapted virus). Surviving animals showed a mixed Th1/Th2 response to the antigen, suggesting this may be important for protection. Survival after vaccination with EIC plus PIC was statistically equivalent to that achieved with an alternative viral vector vaccine candidate reported in the literature. Because nonreplicating subunit vaccines offer the possibility of formulation for cost-effective, long-term storage in biothreat reduction repositories, EIC is an attractive option for public health defense measures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Feldmann H, Klenk HD. Marburg and Ebola viruses. Adv Virus Res. 1996;47:1–52. - PubMed
-
- Jaax N, et al. Transmission of Ebola virus (Zaire strain) to uninfected control monkeys in a biocontainment laboratory. Lancet. 1995;346:1669–1671. - PubMed
-
- Baize S, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. - PubMed
-
- Warfield KL, et al. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol. 2005;175:1184–1191. - PubMed
-
- Wilson JA, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664–1666. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

