Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant
- PMID: 22143789
- PMCID: PMC3251097
- DOI: 10.1073/pnas.1108360108
Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant
Abstract
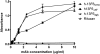
No countermeasures currently exist for the prevention or treatment of the severe sequelae of Filovirus (such as Ebola virus; EBOV) infection. To overcome this limitation in our biodefense preparedness, we have designed monoclonal antibodies (mAbs) which could be used in humans as immunoprotectants for EBOV, starting with a murine mAb (13F6) that recognizes the heavily glycosylated mucin-like domain of the virion-attached glycoprotein (GP). Point mutations were introduced into the variable region of the murine mAb to remove predicted human T-cell epitopes, and the variable regions joined to human constant regions to generate a mAb (h-13F6) appropriate for development for human use. We have evaluated the efficacy of three variants of h-13F6 carrying different glycosylation patterns in a lethal mouse EBOV challenge model. The pattern of glycosylation of the various mAbs was found to correlate to level of protection, with aglycosylated h-13F6 providing the least potent efficacy (ED(50) = 33 μg). A version with typical heterogenous mammalian glycoforms (ED(50) = 11 μg) had similar potency to the original murine mAb. However, h-13F6 carrying complex N-glycosylation lacking core fucose exhibited superior potency (ED(50) = 3 μg). Binding studies using Fcγ receptors revealed enhanced binding of nonfucosylated h-13F6 to mouse and human FcγRIII. Together the results indicate the presence of Fc N-glycans enhances the protective efficacy of h-13F6, and that mAbs manufactured with uniform glycosylation and a higher potency glycoform offer promise as biodefense therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Expression of a Large Single-Chain 13F6 Antibody with Binding Activity against Ebola Virus-Like Particles in a Plant System.Int J Mol Sci. 2020 Sep 23;21(19):7007. doi: 10.3390/ijms21197007. Int J Mol Sci. 2020. PMID: 32977599 Free PMC article.
-
Tobacco against Ebola virus disease.Przegl Lek. 2015;72(10):567-71. Przegl Lek. 2015. PMID: 26946569 Review.
-
Pan-ebolavirus and Pan-filovirus Mouse Monoclonal Antibodies: Protection against Ebola and Sudan Viruses.J Virol. 2015 Oct 14;90(1):266-78. doi: 10.1128/JVI.02171-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468533 Free PMC article.
-
Macaque Monoclonal Antibodies Targeting Novel Conserved Epitopes within Filovirus Glycoprotein.J Virol. 2015 Oct 14;90(1):279-91. doi: 10.1128/JVI.02172-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468532 Free PMC article.
-
The emergence of antibody therapies for Ebola.Hum Antibodies. 2015 Dec 23;23(3-4):49-56. doi: 10.3233/HAB-150284. Hum Antibodies. 2015. PMID: 27472862 Review.
Cited by
-
A Role for Fc Function in Therapeutic Monoclonal Antibody-Mediated Protection against Ebola Virus.Cell Host Microbe. 2018 Aug 8;24(2):221-233.e5. doi: 10.1016/j.chom.2018.07.009. Cell Host Microbe. 2018. PMID: 30092199 Free PMC article.
-
Vaccinal antibodies: Fc antibody engineering to improve the antiviral antibody response and induce vaccine-like effects.Hum Vaccin Immunother. 2021 Dec 2;17(12):5532-5545. doi: 10.1080/21645515.2021.1985891. Epub 2021 Nov 29. Hum Vaccin Immunother. 2021. PMID: 34844516 Free PMC article. Review.
-
Cross-species higher sensitivities of FcγRIIIA/FcγRIV to afucosylated IgG for enhanced ADCC.Antib Ther. 2021 Aug 19;4(3):159-170. doi: 10.1093/abt/tbab016. eCollection 2021 Jul. Antib Ther. 2021. PMID: 34485821 Free PMC article.
-
A Highly Expressing, Soluble, and Stable Plant-Made IgG Fusion Vaccine Strategy Enhances Antigen Immunogenicity in Mice Without Adjuvant.Front Immunol. 2020 Dec 4;11:576012. doi: 10.3389/fimmu.2020.576012. eCollection 2020. Front Immunol. 2020. PMID: 33343565 Free PMC article.
-
Contributions of the international plant science community to the fight against human infectious diseases - part 1: epidemic and pandemic diseases.Plant Biotechnol J. 2021 Oct;19(10):1901-1920. doi: 10.1111/pbi.13657. Epub 2021 Jul 19. Plant Biotechnol J. 2021. PMID: 34182608 Free PMC article. Review.
References
-
- Alibek K. Biohazard: The Chilling True Story of the Largest Covert Biological Weapons Program in the World–Told from Inside by the Man Who Ran It. New York: Random House; 1999.
-
- Wilson JA, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664–1666. - PubMed
-
- Jones TD, Crompton LJ, Carr FJ, Baker MP. Deimmunization of monoclonal antibodies. Methods Mol Biol. 2009;525:405–423, xiv. - PubMed
-
- Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21:11–16. - PubMed
-
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–234. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

