A genome-wide RNAi screen in mouse embryonic stem cells identifies Mp1 as a key mediator of differentiation
- PMID: 22143885
- PMCID: PMC3244037
- DOI: 10.1084/jem.20102037
A genome-wide RNAi screen in mouse embryonic stem cells identifies Mp1 as a key mediator of differentiation
Abstract
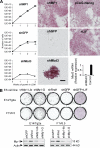
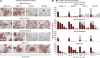
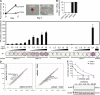
Despite intense investigation of intrinsic and extrinsic factors that regulate pluripotency, the process of initial fate commitment of embryonic stem (ES) cells is still poorly understood. We used a genome-wide short hairpin RNA screen in mouse ES cells to identify genes that are essential for initiation of differentiation. Knockdown of the scaffolding protein Mek binding protein 1 (Mp1, also known as Lamtor3 or Map2k1ip1) stimulated self-renewal of ES cells, blocked differentiation, and promoted proliferation. Fibroblast growth factor 4 (FGF4) signaling is required for initial fate commitment of ES cells. Knockdown of Mp1 inhibited FGF4-induced differentiation but did not alter FGF4-driven proliferation. This uncoupling of differentiation and proliferation was also observed when oncogenic Ras isoforms were overexpressed in ES cells. Knockdown of Mp1 redirected FGF4 signaling from differentiation toward pluripotency and up-regulated the pluripotency-related genes Esrrb, Rex1, Tcl1, and Sox2. We also found that human germ cell tumors (GCTs) express low amounts of Mp1 in the invasive embryonic carcinoma and seminoma histologies and higher amounts of Mp1 in the noninvasive carcinoma in situ precursor and differentiated components. Knockdown of Mp1 in invasive GCT cells resulted in resistance to differentiation, thereby showing a functional role for Mp1 both in normal differentiation of ES cells and in germ cell cancer.
Figures



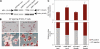
References
-
- Beddington R.S., Robertson E.J. 1989. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 105:733–737 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

