Immature B cells preferentially switch to IgE with increased direct Sμ to Sε recombination
- PMID: 22143888
- PMCID: PMC3244039
- DOI: 10.1084/jem.20111155
Immature B cells preferentially switch to IgE with increased direct Sμ to Sε recombination
Abstract
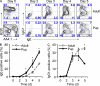
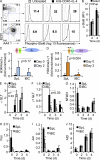
Immunoglobulin heavy chain (IgH) class-switch recombination (CSR) replaces initially expressed Cμ (IgM) constant regions (C(H)) exons with downstream C(H) exons. Stimulation of B cells with anti-CD40 plus interleukin-4 induces CSR from Cμ to Cγ1 (IgG1) and Cε (IgE), the latter of which contributes to the pathogenesis of atopic diseases. Although Cε CSR can occur directly from Cμ, most mature peripheral B cells undergo CSR to Cε indirectly, namely from Cμ to Cγ1, and subsequently to Cε. Physiological mechanisms that influence CSR to Cγ1 versus Cε are incompletely understood. In this study, we report a role for B cell developmental maturity in IgE CSR. Based in part on a novel flow cytometric IgE CSR assay, we show that immature B cells preferentially switch to IgE versus IgG1 through a mechanism involving increased direct CSR from Cμ to Cε. Our findings suggest that IgE dysregulation in certain immunodeficiencies may be related to impaired B cell maturation.
Figures






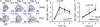

Similar articles
-
Downstream class switching leads to IgE antibody production by B lymphocytes lacking IgM switch regions.Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):3040-5. doi: 10.1073/pnas.0915072107. Epub 2010 Feb 1. Proc Natl Acad Sci U S A. 2010. PMID: 20133637 Free PMC article.
-
B cell Rab7 mediates induction of activation-induced cytidine deaminase expression and class-switching in T-dependent and T-independent antibody responses.J Immunol. 2015 Apr 1;194(7):3065-78. doi: 10.4049/jimmunol.1401896. Epub 2015 Mar 4. J Immunol. 2015. PMID: 25740947 Free PMC article.
-
TRIF signaling is essential for TLR4-driven IgE class switching.J Immunol. 2014 Mar 15;192(6):2651-8. doi: 10.4049/jimmunol.1300909. Epub 2014 Feb 14. J Immunol. 2014. PMID: 24532577 Free PMC article.
-
Molecular Mechanisms of IgE Class Switch Recombination.Curr Top Microbiol Immunol. 2015;388:21-37. doi: 10.1007/978-3-319-13725-4_2. Curr Top Microbiol Immunol. 2015. PMID: 25553793 Review.
-
Evolution of the immunoglobulin heavy chain class switch recombination mechanism.Adv Immunol. 2007;94:157-214. doi: 10.1016/S0065-2776(06)94006-1. Adv Immunol. 2007. PMID: 17560275 Review.
Cited by
-
Primary atopic disorders.J Exp Med. 2018 Apr 2;215(4):1009-1022. doi: 10.1084/jem.20172306. Epub 2018 Mar 16. J Exp Med. 2018. PMID: 29549114 Free PMC article. Review.
-
Unraveling the repertoire in Wiskott-Aldrich syndrome.Front Immunol. 2014 Oct 27;5:539. doi: 10.3389/fimmu.2014.00539. eCollection 2014. Front Immunol. 2014. PMID: 25386182 Free PMC article. Review. No abstract available.
-
IgH isotype-specific B cell receptor expression influences B cell fate.Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):E8411-E8420. doi: 10.1073/pnas.1704962114. Epub 2017 Sep 18. Proc Natl Acad Sci U S A. 2017. PMID: 28923960 Free PMC article.
-
External stimulation induces the secretion of autophagosome-like vesicles by B cells.Autophagy Rep. 2023 Feb 21;2(1):2179287. doi: 10.1080/27694127.2023.2179287. eCollection 2023. Autophagy Rep. 2023. PMID: 40395311 Free PMC article.
-
Regulation of IgE Responses by γδ T Cells.Curr Allergy Asthma Rep. 2015 Apr;15(4):13. doi: 10.1007/s11882-015-0519-z. Curr Allergy Asthma Rep. 2015. PMID: 26130476 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

