Requirement of myeloid cell-specific Fas expression for prevention of systemic autoimmunity in mice
- PMID: 22143975
- PMCID: PMC3290732
- DOI: 10.1002/art.34317
Requirement of myeloid cell-specific Fas expression for prevention of systemic autoimmunity in mice
Abstract
Objective: The death receptor Fas is a critical mediator of the extrinsic apoptotic pathway, and its role in mediating lymphoproliferation has been extensively examined. The present study was undertaken to investigate the impact of myeloid cell-specific loss of Fas.
Methods: Mice with Fas flanked by loxP sites (Fas(flox/flox) ) were crossed with mice expressing Cre under control of the murine lysozyme M gene promoter (Cre(LysM) ), which functions in mature lysozyme-expressing cells of the myelomonocytic lineage. The genotype for Cre(LysM) Fas(flox/flox) mice was verified by polymerase chain reaction and flow cytometric analysis. Flow cytometric analysis was also used to characterize myeloid, dendritic, and lymphoid cell distribution and activation in bone marrow, blood, and spleen. Luminex-based assays and enzyme-linked immunosorbent assays were used to measure serum cytokine/chemokine and immunoglobulin levels. Renal damage or dysfunction was examined by immunohistochemical and immunofluorescence analysis.
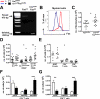
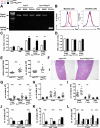
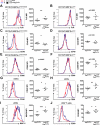
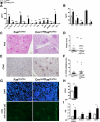
Results: Cre(LysM) Fas(flox/flox) mice exhibited a systemic lupus erythematosus (SLE)-like disease that included leukocytosis, splenomegaly, hypergammaglobulinemia, antinuclear autoantibody and proinflammatory cytokine production, and glomerulonephritis. Loss of Fas in myeloid cells increased levels of both Gr-1(low) and Gr-1(intermediate) blood monocytes and splenic macrophages and, in a paracrine manner, incited activation of conventional dendritic cells and lymphocytes in Cre(LysM) Fas(flox/flox) mice.
Conclusion: Taken together, these results suggest that loss of Fas in myeloid cells is sufficient to induce inflammatory phenotypes in mice, reminiscent of an SLE-like disease. Thus, Fas in myeloid cells may be considered a suppressor of systemic autoimmunity.
Copyright © 2012 by the American College of Rheumatology.
Figures


Comment in
-
Systemic autoimmunity caused by fas deficiency in macrophages: a new perspective on the first identified autoimmunity gene.Arthritis Rheum. 2012 Mar;64(3):609-12. doi: 10.1002/art.34321. Arthritis Rheum. 2012. PMID: 22139895 No abstract available.
References
-
- Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AR055503/AR/NIAMS NIH HHS/United States
- AI-039824/AI/NIAID NIH HHS/United States
- R21 AI067590/AI/NIAID NIH HHS/United States
- AR-054796/AR/NIAMS NIH HHS/United States
- AR-060169/AR/NIAMS NIH HHS/United States
- AR-050250/AR/NIAMS NIH HHS/United States
- R01 AR050250/AR/NIAMS NIH HHS/United States
- R01 AR054796/AR/NIAMS NIH HHS/United States
- F32 AR060169/AR/NIAMS NIH HHS/United States
- R01 AR055600/AR/NIAMS NIH HHS/United States
- AR-050812/AR/NIAMS NIH HHS/United States
- P01 AI039824/AI/NIAID NIH HHS/United States
- AI-067590/AI/NIAID NIH HHS/United States
- AR-055240/AR/NIAMS NIH HHS/United States
- AR-055600/AR/NIAMS NIH HHS/United States
- R01 AR055240/AR/NIAMS NIH HHS/United States
- R01 AR050812/AR/NIAMS NIH HHS/United States
- AR-055503/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

