Astrocyte stellation, a process dependent on Rac1 is sustained by the regulated exocytosis of enlargeosomes
- PMID: 22144092
- PMCID: PMC3306795
- DOI: 10.1002/glia.22280
Astrocyte stellation, a process dependent on Rac1 is sustained by the regulated exocytosis of enlargeosomes
Abstract
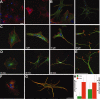
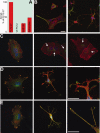
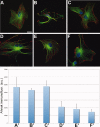
Cultured astrocytes exhibit a flat/epitelioid phenotype much different from the star-like phenotype of tissue astrocytes. Upon exposure to treatments that affect the small GTPase Rho and/or its effector ROCK, however, flat astrocytes undergo stellation, with restructuring of cytoskeleton and outgrowth of processes with lamellipodia, assuming a phenotype closer to that exhibited in situ. The mechanisms of this change are known only in part. Using the ROCK blocker drug Y27632, which induces rapid (tens of min), dose-dependent and reversible stellations, we focused on two specific aspects of the process: its dependence on small GTPases and the large surface expansion of the cells. Contrary to previous reports, we found stellation to be governed by the small G protein Rac1, up to disappearance of the process when Rac1 was downregulated or blocked by a specific drug. In contrast cdc42, the other G-protein often involved in phenotype changes, appeared not involved. The surface expansion concomitant to cytoskeleton restructuring, also dependent on Rac1, was found to be at least partially sustained by the exocytosis of enlargeosomes, small vesicles distinct from classical cell organelles, which are abundant in astrocytes. Exhaustion of stellation induced by repeated administrations of Y27632 correlated with the decrease of the enlargeosome pool. A whole-cell process like stellation of cultured astrocytes might be irrelevant in the brain tissue. However, local restructuring of the cytoskeleton coordinate with surface expansion, occurring at critical cell sites and sustained by mechanisms analogous to those of stellation, might be of importance in both astrocyte physiology and pathology.
Copyright © 2011 Wiley Periodicals, Inc.
Figures



Similar articles
-
Rapid neurite outgrowth in neurosecretory cells and neurons is sustained by the exocytosis of a cytoplasmic organelle, the enlargeosome.J Cell Sci. 2010 Jan 15;123(Pt 2):165-70. doi: 10.1242/jcs.059634. Epub 2009 Dec 21. J Cell Sci. 2010. PMID: 20026640
-
Role of Rho GTPase in astrocyte morphology and migratory response during in vitro wound healing.J Neurochem. 2005 Dec;95(5):1237-48. doi: 10.1111/j.1471-4159.2005.03443.x. Epub 2005 Sep 7. J Neurochem. 2005. PMID: 16150054
-
N-myc downstream-regulated gene 2 controls astrocyte morphology via Rho-GTPase signaling.J Cell Physiol. 2019 Nov;234(11):20847-20858. doi: 10.1002/jcp.28689. Epub 2019 Apr 19. J Cell Physiol. 2019. PMID: 31004356
-
CDK5 knockdown in astrocytes provide neuroprotection as a trophic source via Rac1.Mol Cell Neurosci. 2015 Sep;68:151-66. doi: 10.1016/j.mcn.2015.07.001. Epub 2015 Jul 6. Mol Cell Neurosci. 2015. PMID: 26160434
-
Non-secretory exocytoses in the brain.J Physiol Paris. 2006 Mar-May;99(2-3):140-5. doi: 10.1016/j.jphysparis.2005.12.081. Epub 2006 Jan 19. J Physiol Paris. 2006. PMID: 16426824 Review.
Cited by
-
Biology of Glioblastoma Multiforme-Exploration of Mitotic Catastrophe as a Potential Treatment Modality.Int J Mol Sci. 2020 Jul 27;21(15):5324. doi: 10.3390/ijms21155324. Int J Mol Sci. 2020. PMID: 32727112 Free PMC article. Review.
-
Vav3-Deficient Astrocytes Enhance the Dendritic Development of Hippocampal Neurons in an Indirect Co-culture System.Front Cell Neurosci. 2022 Feb 14;15:817277. doi: 10.3389/fncel.2021.817277. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35237130 Free PMC article.
-
Two Metabolic Fuels, Glucose and Lactate, Differentially Modulate Exocytotic Glutamate Release from Cultured Astrocytes.Neurochem Res. 2021 Oct;46(10):2551-2579. doi: 10.1007/s11064-021-03340-y. Epub 2021 May 31. Neurochem Res. 2021. PMID: 34057673 Free PMC article.
-
Astrocytes in Chronic Pain: Cellular and Molecular Mechanisms.Neurosci Bull. 2023 Mar;39(3):425-439. doi: 10.1007/s12264-022-00961-3. Epub 2022 Nov 14. Neurosci Bull. 2023. PMID: 36376699 Free PMC article. Review.
-
Metabolic Dysfunction of Astrocyte: An Initiating Factor in Beta-amyloid Pathology?Aging Neurodegener. 2013 Aug;1(1):7-14. Aging Neurodegener. 2013. PMID: 24443714 Free PMC article.
References
-
- Abe K, Misawa M. Astrocyte stellation induced by Rho kinase inhibitors in culture. Dev Brain Res. 2003;143:99–104. - PubMed
-
- Boran MS, Garcia A. The cyclic GMP-protein kinase G pathway regulates cytoskeleton dynamics and motility of astrocytes. J Neurochem. 2007;102:216–230. - PubMed
-
- Borgonovo B, Cocucci E, Racchetti G, Podini P, Bachi A, Meldolesi J. Regulated exocytosis: A novel, widely expressed system. Nat Cell Biol. 2002;4:955–962. - PubMed
-
- Chao TI, Rickmann M, Wolff JR. The synapse-astrocyte boundary: An anatomical basis for an integrative role of glia in synaptic transmission. In: Volterra A, et al., editors. The tripartite synapse: Glia in synaptic transmission. Oxford: Oxford University Press; 2002. pp. 3–23.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

