Expression of paramyxovirus V proteins promotes replication and spread of hepatitis C virus in cultures of primary human fetal liver cells
- PMID: 22144107
- PMCID: PMC3233237
- DOI: 10.1002/hep.24557
Expression of paramyxovirus V proteins promotes replication and spread of hepatitis C virus in cultures of primary human fetal liver cells
Abstract
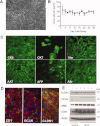
Here we demonstrate that primary cultures of human fetal liver cells (HFLC) reliably support infection with laboratory strains of hepatitis C virus (HCV), although levels of virus replication vary significantly between different donor cell preparations and frequently decline in a manner suggestive of active viral clearance. To investigate possible contributions of the interferon (IFN) system to control HCV infection in HFLC, we exploited the well-characterized ability of paramyxovirus (PMV) V proteins to counteract both IFN induction and antiviral signaling. The V proteins of measles virus (MV) and parainfluenza virus 5 (PIV5) were introduced into HFLC using lentiviral vectors encoding a fluorescent reporter for visualization of HCV-infected cells. V protein-transduced HFLC supported enhanced (10 to 100-fold) levels of HCV infection relative to untransduced or control vector-transduced HFLC. Infection was assessed by measurement of virus-driven luciferase, by assays for infectious HCV and viral RNA, and by direct visualization of HCV-infected hepatocytes. Live cell imaging between 48 and 119 hours postinfection demonstrated little or no spread of infection in the absence of PMV V protein expression. In contrast, V protein-transduced HFLC showed numerous HCV infection events. V protein expression efficiently antagonized the HCV-inhibitory effects of added IFNs in HFLC. In addition, induction of the type III IFN, IL29, following acute HCV infection was inhibited in V protein-transduced cultures.
Conclusion: These studies suggest that the cellular IFN response plays a significant role in limiting the spread of HCV infection in primary hepatocyte cultures. Strategies aimed at dampening this response may be key to further development of robust HCV culture systems, enabling studies of virus pathogenicity and the mechanisms by which HCV spreads in its natural host cell population.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures




References
-
- Altar HJ, Seeff LB. Recovery, persistence and sequelae in hepatitis C virus infection: a perspective on the long-term outcome. Semin Liv Dis. 2000;20:17–25. - PubMed
-
- Otsuka M, Kato N, Moriyama M, Taniguchi H, Wang Y, Dharel N, et al. Interaction between the HCV NS3 protein and the host TBK1 protein leads to inhibition of cellular antiviral responses. HEPATOLOGY. 2005;41:1004–1012. - PubMed
-
- Gale M, Foy EM. Evasion of intracellular host defense by hepatitis C virus. Nature. 2005;436:939–945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
