Development and evaluation of a rapid diagnostic test for Plasmodium falciparum, P. vivax, and mixed-species malaria antigens
- PMID: 22144432
- PMCID: PMC3225176
- DOI: 10.4269/ajtmh.2011.11-0265
Development and evaluation of a rapid diagnostic test for Plasmodium falciparum, P. vivax, and mixed-species malaria antigens
Abstract
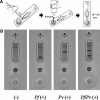
Plasmodium falciparum and P. vivax malaria are endemic to many parts of the world and humans can be co-infected with both species. Because each Plasmodium species has different biological and clinical characteristics, accurate differentiation of the infecting species is essential for effective treatment. Therefore, we produced three monoclonal antibodies that recognize the lactate dehydrogenase of P. falciparum, P. vivax, or both to develop the first P. falciparum, P. vivax, and mixed-species infections malaria antigen detection kit. The detection limits of this kit were 150 and 250 parasites/μL for P. falciparum and P. vivax, respectively, and the kit was able to detect mixed-species infections. The sensitivity and specificity of this kit was assessed with 722 clinical specimens. Our results showed that its sensitivities for P. falciparum, P. vivax, and mixed-species infection were 96.5%, 95.3%, and 85.7%, respectively. In addition, its specificity was high (99.4%).
Figures

References
-
- Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–240. - PubMed
-
- Siribal S, Nakasiri S, Looareesuwan S, Chavalitshewinkoon-Petmitr P. Identification of human malaria parasites and detection of mixed infection in Thai patients by nested PCR. Southeast Asian J Trop Med Public Health. 2004;35:5–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

