Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women
- PMID: 22144471
- PMCID: PMC3271178
- DOI: 10.1158/0008-5472.CAN-11-2507
Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women
Abstract
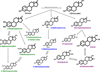
Endogenous estrogens and estrogen metabolism are hypothesized to be associated with premenopausal breast cancer risk but evidence is limited. We examined 15 urinary estrogens/estrogen metabolites and breast cancer risk among premenopausal women in a case-control study nested within the Nurses' Health Study II (NHSII). From 1996 to 1999, urine was collected from 18,521 women during the mid-luteal menstrual phase. Breast cancer cases (N = 247) diagnosed between collection and June 2005 were matched to two controls each (N = 485). Urinary estrogen metabolites were measured by liquid chromatography-tandem mass spectrometry and adjusted for creatinine level. Relative risks (RR) and 95% confidence intervals (CI) were estimated by multivariate conditional logistic regression. Higher urinary estrone and estradiol levels were strongly significantly associated with lower risk (top vs. bottom quartile RR: estrone = 0.52; 95% CI, 0.30-0.88; estradiol = 0.51; 95% CI, 0.30-0.86). Generally inverse, although nonsignificant, patterns also were observed with 2- and 4-hydroxylation pathway estrogen metabolites. Inverse associations generally were not observed with 16-pathway estrogen metabolites and a significant positive association was observed with 17-epiestriol (top vs. bottom quartile RR = 1.74; 95% CI, 1.08-2.81; P(trend) = 0.01). In addition, there was a significant increased risk with higher 16-pathway/parent estrogen metabolite ratio (comparable RR = 1.61; 95% CI, 0.99-2.62; P(trend) = 0.04). Other pathway ratios were not significantly associated with risk except parent estrogen metabolites/non-parent estrogen metabolites (comparable RR = 0.58; 95% CI, 0.35-0.96; P(trend) = 0.03). These data suggest that most mid-luteal urinary estrogen metabolite concentrations are not positively associated with breast cancer risk among premenopausal women. The inverse associations with parent estrogen metabolites and the parent estrogen metabolite/non-parent estrogen metabolite ratio suggest that women with higher urinary excretion of parent estrogens are at lower risk.
©2011 AACR.
Figures



References
-
- Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. - PubMed
-
- Wysowski DK, Comstock GW, Helsing KJ, Lau HL. Sex hormone levels in serum in relation to the development of breast cancer. Am J Epidemiol. 1987;125:791–799. - PubMed
-
- Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev. 1994;18:79–85. - PubMed
-
- Rosenberg CR, Pasternack BS, Shore RE, Koenig KL, Toniolo PG. Premenopausal estradiol levels and the risk of breast cancer: a new method of controlling for day of the menstrual cycle. Am J Epidemiol. 1994;140:518–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

