Bacillus subtilis-mediated protection from Citrobacter rodentium-associated enteric disease requires espH and functional flagella
- PMID: 22144475
- PMCID: PMC3264298
- DOI: 10.1128/IAI.05843-11
Bacillus subtilis-mediated protection from Citrobacter rodentium-associated enteric disease requires espH and functional flagella
Abstract
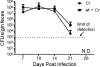
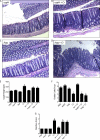
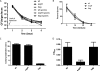
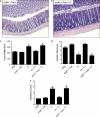
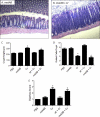
Commensals limit disease caused by invading pathogens; however, the mechanisms and genes utilized by beneficial microbes to inhibit pathogenesis are poorly understood. The attaching and effacing mouse pathogen Citrobacter rodentium associates intimately with the intestinal epithelium, and infections result in acute colitis. C. rodentium is used to model the human pathogens enterohemorrhagic Escherichia coli and enteropathogenic E. coli. To confirm that Bacillus subtilis, a spore-forming bacterium found in the gut of mammals, could reduce C. rodentium-associated disease, mice received wild-type B. subtilis spores and 24 h later were infected by oral gavage with pathogenic C. rodentium. Disease was assessed by determining the extent of colonic epithelial hyperplasia, goblet cell loss, diarrhea, and pathogen colonization. Mice that received wild-type B. subtilis prior to enteric infection were protected from disease even though C. rodentium colonization was not inhibited. In contrast, espH and hag mutants, defective in exopolysaccharides and flagellum production, respectively, did not protect mice from C. rodentium-associated disease. A motAB mutant also failed to protect mice from disease, suggesting that B. subtilis-mediated protection requires functional flagella. By expanding our current mechanistic knowledge of bacterial protection, we can better utilize beneficial microbes to prevent intestinal disease caused by pathogenic bacteria, ultimately reducing human disease. Our data demonstrate that wild-type B. subtilis reduced disease caused by C. rodentium infection through a mechanism that required espH and functional flagella.
Figures

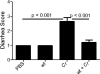
Similar articles
-
Bacillus subtilis spores reduce susceptibility to Citrobacter rodentium-mediated enteropathy in a mouse model.Res Microbiol. 2006 Nov;157(9):891-7. doi: 10.1016/j.resmic.2006.06.001. Epub 2006 Sep 26. Res Microbiol. 2006. PMID: 17005378
-
Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen.Infect Immun. 2008 Feb;76(2):796-811. doi: 10.1128/IAI.00093-07. Epub 2007 Nov 5. Infect Immun. 2008. PMID: 17984203 Free PMC article.
-
Intestinal Epithelial Cells and the Microbiome Undergo Swift Reprogramming at the Inception of Colonic Citrobacter rodentium Infection.mBio. 2019 Apr 2;10(2):e00062-19. doi: 10.1128/mBio.00062-19. mBio. 2019. PMID: 30940698 Free PMC article.
-
Citrobacter rodentium of mice and man.Cell Microbiol. 2005 Dec;7(12):1697-706. doi: 10.1111/j.1462-5822.2005.00625.x. Cell Microbiol. 2005. PMID: 16309456 Review.
-
Attaching and effacing pathogens modulate host mitochondrial structure and function.Int Rev Cell Mol Biol. 2023;377:65-86. doi: 10.1016/bs.ircmb.2023.03.001. Epub 2023 Apr 3. Int Rev Cell Mol Biol. 2023. PMID: 37268351 Free PMC article. Review.
Cited by
-
EPEC effector EspF promotes Crumbs3 endocytosis and disrupts epithelial cell polarity.Cell Microbiol. 2017 Nov;19(11):10.1111/cmi.12757. doi: 10.1111/cmi.12757. Epub 2017 Jul 27. Cell Microbiol. 2017. PMID: 28618099 Free PMC article.
-
Fermented dairy products modulate Citrobacter rodentium-induced colonic hyperplasia.J Infect Dis. 2014 Oct 1;210(7):1029-41. doi: 10.1093/infdis/jiu205. Epub 2014 Apr 4. J Infect Dis. 2014. PMID: 24706936 Free PMC article.
-
Adjuvant conditioning shapes the adaptive immune response promoting immunotolerance via NLRP3/interleukin-1.iScience. 2025 May 13;28(6):112653. doi: 10.1016/j.isci.2025.112653. eCollection 2025 Jun 20. iScience. 2025. PMID: 40530419 Free PMC article.
-
Probiotic Exopolysaccharide Protects against Systemic Staphylococcus aureus Infection, Inducing Dual-Functioning Macrophages That Restrict Bacterial Growth and Limit Inflammation.Infect Immun. 2018 Dec 19;87(1):e00791-18. doi: 10.1128/IAI.00791-18. Print 2019 Jan. Infect Immun. 2018. PMID: 30396894 Free PMC article.
-
Protection from intestinal inflammation by bacterial exopolysaccharides.J Immunol. 2014 May 15;192(10):4813-20. doi: 10.4049/jimmunol.1303369. Epub 2014 Apr 16. J Immunol. 2014. PMID: 24740503 Free PMC article.
References
-
- Borenshtein D, McBee ME, Schauer DB. 2008. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr. Opin. Gastroenterol. 24:32–37 - PubMed
-
- Castagliuolo I, et al. 2005. Beneficial effect of auto-aggregating Lactobacillus crispatus on experimentally induced colitis in mice. FEMS Immunol. Med. Microbiol. 43:197–204 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

