Differential gene expression in mice infected with distinct Toxoplasma strains
- PMID: 22144491
- PMCID: PMC3294647
- DOI: 10.1128/IAI.05421-11
Differential gene expression in mice infected with distinct Toxoplasma strains
Abstract
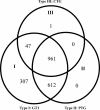
Toxoplasma gondii is the causative agent of toxoplasmosis in human and animals. In a mouse model, T. gondii strains can be divided into three groups, including the virulent, intermediately virulent, and nonvirulent. The clonal type I, II, and III T. gondii strains belong to these three groups, respectively. To better understand the basis of virulence phenotypes, we investigated mouse gene expression responses to the infection of different T. gondii strains at day 5 after intraperitoneal inoculation with 500 tachyzoites. The transcriptomes of mouse peritoneal cells showed that 1,927, 1,573, and 1,009 transcripts were altered more than 2-fold by type I, II, and III infections, respectively, and that the majority of altered transcripts were shared. Overall transcription patterns were similar in type I and type II infections, and both had greater changes than infection with type III. Quantification of parasite burden in mouse spleens showed that the burden with type I infection was 1,000 times higher than that of type II and that the type II burden was 20 times higher than that of type III. Fluorescence-activated cell sorting revealed that type I and II infections had comparable macrophage populations, and both were higher than the population with type III infection. In addition, type I infection had a higher percentage of neutrophils than type II and III infections. Taken together, these results suggested that there is a common gene expression response to T. gondii infection in mice. This response is further modified by parasite strain-specific factors that determine their distinct virulence phenotypes.
Figures




References
-
- Ahn HJ, Kim JY, Ryu K, Nam HW. 2009. STAT6 activation by Toxoplasma gondii infection induces the expression of Th2 C-C chemokine ligands and B clade serine protease inhibitors in macrophage. Parasitol. Res. 105:1445–1453 - PubMed
-
- Ansel KM, Harris RBS, Cyster JG. 2002. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16:67–76 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

