Small intestinal intraepithelial lymphocytes expressing CD8 and T cell receptor γδ are involved in bacterial clearance during Salmonella enterica serovar Typhimurium infection
- PMID: 22144492
- PMCID: PMC3264294
- DOI: 10.1128/IAI.05078-11
Small intestinal intraepithelial lymphocytes expressing CD8 and T cell receptor γδ are involved in bacterial clearance during Salmonella enterica serovar Typhimurium infection
Abstract
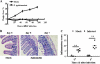
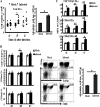
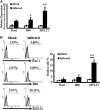
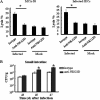
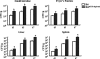
The intestinal immune system is crucial for the maintenance of mucosal homeostasis and has evolved under the dual pressure of protecting the host from pathogenic infection and coexisting with the dense and diverse commensal organisms in the lumen. Intestinal intraepithelial lymphocytes (iIELs) are the first element of the host T cell compartment available to respond to oral infection by pathogens. This study demonstrated that oral infection by Salmonella enterica serovar Typhimurium promoted the expansion of iIELs, particularly CD8(+) TCRγδ(+) IELs, enhanced expression of NKG2D on iIELs, increased expression of MULT1, and decreased expression of Qa-1 by intestinal epithelial cells (IECs), leading to activation of, particularly, CD8(+) TCRγδ(+) iIELs and cytolytic activity against S. Typhimurium-infected IECs. Blockade of NKG2D recognition or depletion of TCRγδ(+) cells using a depleting monoclonal antibody significantly attenuated the clearance of S. Typhimurium in the intestine and other tissues. This study suggests that iIELs, particularly CD8(+) TCRγδ(+) iIELs, play important roles in the detection of pathogenic bacteria and eradication of infected epithelial cells and, thus, provide protection against invading pathogens. These data further our understanding of the mechanisms by which the immune system of the intestinal mucosa discriminates between pathogenic and commensal organisms.
Figures


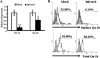
References
-
- Carding SR, Egan PJ. 2002. γδT cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2:336–345 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

