Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations
- PMID: 22144569
- PMCID: PMC3276214
- DOI: 10.1161/CIRCULATIONAHA.111.045096
Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations
Abstract
Background: Migraine is an independent risk factor for stroke. Mechanisms underlying this association are unclear. Familial hemiplegic migraine (FHM), a migraine subtype that also carries an increased stroke risk, is a useful model for common migraine phenotypes because of shared aura and headache features, trigger factors, and underlying glutamatergic mechanisms.
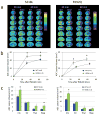
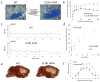
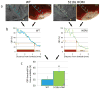
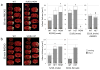
Methods and results: Here, we show that FHM type 1 (FHM1) mutations in Ca(V)2.1 voltage-gated Ca(2+) channels render the brain more vulnerable to ischemic stroke. Compared with wild-type mice, 2 FHM1 mutant mouse strains developed earlier onset of anoxic depolarization and more frequent peri-infarct depolarizations associated with rapid expansion of infarct core on diffusion-weighted magnetic resonance imaging and larger perfusion deficits on laser speckle flowmetry. Cerebral blood flow required for tissue survival was higher in the mutants, leading to infarction with milder ischemia. As a result, mutants developed larger infarcts and worse neurological outcomes after stroke, which were selectively attenuated by a glutamate receptor antagonist.
Conclusions: We propose that enhanced susceptibility to ischemic depolarizations akin to spreading depression predisposes migraineurs to infarction during mild ischemic events, thereby increasing the stroke risk.
© 2011 American Heart Association, Inc.
Conflict of interest statement
Figures
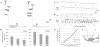
References
-
- Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD, Launer LJ. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291:427–434. - PubMed
-
- Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain. 2002;125:1379–1391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS061505/NS/NINDS NIH HHS/United States
- NS057556/NS/NINDS NIH HHS/United States
- R01 DA026108/DA/NIDA NIH HHS/United States
- NS35611/NS/NINDS NIH HHS/United States
- R21 DA024235/DA/NIDA NIH HHS/United States
- DA024235/DA/NIDA NIH HHS/United States
- R21 NS057556/NS/NINDS NIH HHS/United States
- R01 NS061505/NS/NINDS NIH HHS/United States
- NS055104/NS/NINDS NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- P01 NS055104/NS/NINDS NIH HHS/United States
- DA026108/DA/NIDA NIH HHS/United States
- P01 NS035611/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

