Hedgehog signalling in gut development, physiology and cancer
- PMID: 22144577
- PMCID: PMC3379690
- DOI: 10.1113/jphysiol.2011.220681
Hedgehog signalling in gut development, physiology and cancer
Abstract
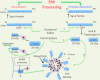
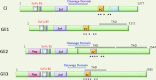
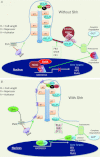
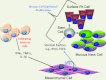
The Hedgehog pathway is one of the most common signal transduction pathways used by mammalian cells. Most studies have focused on its role during development, primarily of the nervous system, skin, bone and pancreas. Due to the activation of this pathway during proliferation and neoplastic transformation, more recent studies have examined its role in adult tissues. Significant levels of sonic hedgehog are expressed in the gastric mucosa, which has served to direct analysis of its role during organogenesis, gastric acid secretion and neoplastic transformation. Therefore the goal of this review is to apply current knowledge of this pathway to further our understanding of gastrointestinal physiology and neoplasia, using the stomach as a prototype.
Figures

Similar articles
-
Hedgehog signaling and gastrointestinal cancer.Biochim Biophys Acta. 2010 Jul;1803(7):786-95. doi: 10.1016/j.bbamcr.2010.03.008. Epub 2010 Mar 19. Biochim Biophys Acta. 2010. PMID: 20307590 Free PMC article. Review.
-
Hedgehog signaling in development and homeostasis of the gastrointestinal tract.Physiol Rev. 2007 Oct;87(4):1343-75. doi: 10.1152/physrev.00054.2006. Physiol Rev. 2007. PMID: 17928586 Review.
-
Hedgehog signalling pathway activation in gastrointestinal stromal tumours is mediated by primary cilia.Gastric Cancer. 2020 Jan;23(1):64-72. doi: 10.1007/s10120-019-00984-2. Epub 2019 Jul 2. Gastric Cancer. 2020. PMID: 31267361
-
The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease.Gastroenterology. 2005 Nov;129(5):1696-710. doi: 10.1053/j.gastro.2005.05.010. Gastroenterology. 2005. PMID: 16285967 Review.
-
Activation of Hedgehog pathway in gastrointestinal cancers.Vitam Horm. 2012;88:461-72. doi: 10.1016/B978-0-12-394622-5.00020-1. Vitam Horm. 2012. PMID: 22391316 Review.
Cited by
-
Recapitulating Human Gastric Cancer Pathogenesis: Experimental Models of Gastric Cancer.Adv Exp Med Biol. 2016;908:441-78. doi: 10.1007/978-3-319-41388-4_22. Adv Exp Med Biol. 2016. PMID: 27573785 Free PMC article. Review.
-
Hedgehog signaling in gastrointestinal carcinogenesis and the gastrointestinal tumor microenvironment.Acta Pharm Sin B. 2021 Mar;11(3):609-620. doi: 10.1016/j.apsb.2020.10.022. Epub 2020 Oct 29. Acta Pharm Sin B. 2021. PMID: 33777671 Free PMC article. Review.
-
Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia.J Clin Invest. 2016 Aug 1;126(8):2867-80. doi: 10.1172/JCI82529. Epub 2016 Jul 18. J Clin Invest. 2016. PMID: 27427984 Free PMC article.
-
Plasmacytoid Dendritic Cell-Derived Type I Interferon Is Involved in Helicobacter pylori Infection-Induced Differentiation of Schlafen 4-Expressing Myeloid-Derived Suppressor Cells.Infect Immun. 2021 Oct 15;89(11):e0040721. doi: 10.1128/IAI.00407-21. Epub 2021 Aug 2. Infect Immun. 2021. PMID: 34370509 Free PMC article.
-
Sonic hedgehog antagonists reduce size and alter patterning of the frog inner ear.Dev Neurobiol. 2017 Dec;77(12):1385-1400. doi: 10.1002/dneu.22544. Epub 2017 Oct 24. Dev Neurobiol. 2017. PMID: 29030893 Free PMC article.
References
-
- Adolphe C, Narang M, Ellis T, Wicking C, Kaur P, Wainwright B. An in vivo comparative study of sonic, desert and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development. 2004;131:5009–5019. - PubMed
-
- Alinger B, Kiesslich T, Datz C, Aberger F, Strasser F, Berr F, Dietze O, Kaserer K, Hauser-Kronberger C. Hedgehog signaling is involved in differentiation of normal colonic tissue rather than in tumor proliferation. Virchows Arch. 2009;454:369–379. - PubMed
-
- Beug ST, Parks RJ, McBride HM, Wallace VA. Processing-dependent trafficking of Sonic hedgehog to the regulated secretory pathway in neurons. Mol Cell Neurosci. 2011;46:583–596. - PubMed

