Amyloid precursor protein revisited: neuron-specific expression and highly stable nature of soluble derivatives
- PMID: 22144675
- PMCID: PMC3268404
- DOI: 10.1074/jbc.M111.315051
Amyloid precursor protein revisited: neuron-specific expression and highly stable nature of soluble derivatives
Abstract
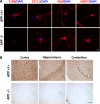
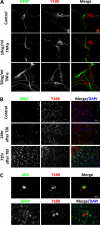
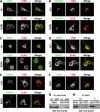
APP processing and amyloid-β production play a central role in Alzheimer disease pathogenesis. APP has been considered a ubiquitously expressed protein. In addition to amyloid-β, α- or β-secretase-dependent cleavage of APP also generates soluble secreted APP (APPsα or APPsβ, respectively). Interestingly, APPsβ has been shown to be subject to further cleavage to create an N-APP fragment that binds to the DR6 death receptor and mediates axon pruning and degeneration under trophic factor withdrawal conditions. By performing APP immunocytochemical staining, we found that, unexpectedly, many antibodies yielded nonspecific staining in APP-null samples. Screening of a series of antibodies allowed us to identify a rabbit monoclonal antibody Y188 that is highly specific for APP and prompted us to re-examine the expression, localization, and stability of endogenous APP and APPsβ in wild-type and in APPsβ knock-in mice, respectively. In contrast to earlier studies, we found that APP is specifically expressed in neurons and that its expression cannot be detected in major types of glial cells under basal or neuroinflammatory conditions. Both APPsα and APPsβ are highly stable in the central nervous system (CNS) and do not undergo further cleavage with or without trophic factor support. Our results clarify several key questions with regard to the fundamental properties of APP and offer critical cellular insights into the pathophysiology of APP.
Figures


References
-
- Chauvet N., Apert C., Dumoulin A., Epelbaum J., Alonso G. (1997) Mab22C11 antibody to amyloid precursor protein recognizes a protein associated with specific astroglial cells of the rat central nervous system characterized by their capacity to support axonal outgrowth. J. Comp. Neurol. 377, 550–564 - PubMed
-
- LeBlanc A. C., Papadopoulos M., Bélair C., Chu W., Crosato M., Powell J., Goodyer C. G. (1997) Processing of amyloid precursor protein in human primary neuron and astrocyte cultures. J. Neurochem. 68, 1183–1190 - PubMed
-
- Rohan de Silva H. A., Jen A., Wickenden C., Jen L. S., Wilkinson S. L., Patel A. J. (1997) Cell-specific expression of β-amyloid precursor protein isoform mRNAs and proteins in neurons and astrocytes. Brain Res. Mol. Brain Res. 47, 147–156 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

