Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans
- PMID: 22144890
- PMCID: PMC3228790
- DOI: 10.1371/journal.ppat.1002389
Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans
Abstract
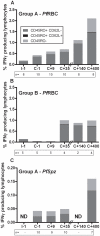
Cellular responses to Plasmodium falciparum parasites, in particular interferon-gamma (IFNγ) production, play an important role in anti-malarial immunity. However, clinical immunity to malaria develops slowly amongst naturally exposed populations, the dynamics of cellular responses in relation to exposure are difficult to study and data about the persistence of such responses are controversial. Here we assess the longevity and composition of cellular immune responses following experimental malaria infection in human volunteers. We conducted a longitudinal study of cellular immunological responses to sporozoites (PfSpz) and asexual blood-stage (PfRBC) malaria parasites in naïve human volunteers undergoing single (n = 5) or multiple (n = 10) experimental P. falciparum infections under highly controlled conditions. IFNγ and interleukin-2 (IL-2) responses following in vitro re-stimulation were measured by flow-cytometry prior to, during and more than one year post infection. We show that cellular responses to both PfSpz and PfRBC are induced and remain almost undiminished up to 14 months after even a single malaria episode. Remarkably, not only 'adaptive' but also 'innate' lymphocyte subsets contribute to the increased IFNγ response, including αβT cells, γδT cells and NK cells. Furthermore, results from depletion and autologous recombination experiments of lymphocyte subsets suggest that immunological memory for PfRBC is carried within both the αβT cells and γδT compartments. Indeed, the majority of cytokine producing T lymphocytes express an CD45RO(+) CD62L(-) effector memory (EM) phenotype both early and late post infection. Finally, we demonstrate that malaria infection induces and maintains polyfunctional (IFNγ(+)IL-2(+)) EM responses against both PfRBC and PfSpz, previously found to be associated with protection. These data demonstrate that cellular responses can be readily induced and are long-lived following infection with P. falciparum, with a persisting contribution by not only adaptive but also (semi-)innate lymphocyte subsets. The implications hereof are positive for malaria vaccine development, but focus attention on those factors potentially inhibiting such responses in the field.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Memory-like IFN-γ response by NK cells following malaria infection reveals the crucial role of T cells in NK cell activation by P. falciparum.Eur J Immunol. 2010 Dec;40(12):3472-7. doi: 10.1002/eji.201040587. Eur J Immunol. 2010. PMID: 21072880
-
Selected P. falciparum specific immune responses are maintained in AIDS adults in Burkina Faso.Parasite Immunol. 1996 Jul;18(7):333-9. doi: 10.1046/j.1365-3024.1996.d01-116.x. Parasite Immunol. 1996. PMID: 9229386
-
Detection of CD4+CD45RO+ T lymphocytes producing IL-4 in response to antigens on Plasmodium falciparum erythrocytes: an in vitro correlate of protective immunity induced with attenuated Plasmodium falciparum sporozoites.Cell Immunol. 1997 Sep 15;180(2):143-52. doi: 10.1006/cimm.1997.1186. Cell Immunol. 1997. PMID: 9341744
-
Immune activation and induction of memory: lessons learned from controlled human malaria infection with Plasmodium falciparum.Parasitology. 2016 Feb;143(2):224-35. doi: 10.1017/S0031182015000761. Parasitology. 2016. PMID: 26864135 Review.
-
[Immunology of human Plasmodium falciparum malaria].Ann Soc Belg Med Trop. 1995 Sep;75(3):159-78. Ann Soc Belg Med Trop. 1995. PMID: 8849294 Review. French.
Cited by
-
A diversified role for γδT cells in vector-borne diseases.Front Immunol. 2022 Aug 16;13:965503. doi: 10.3389/fimmu.2022.965503. eCollection 2022. Front Immunol. 2022. PMID: 36052077 Free PMC article. Review.
-
Setting the stage: The initial immune response to blood-stage parasites.Virulence. 2020 Dec;11(1):88-103. doi: 10.1080/21505594.2019.1708053. Virulence. 2020. PMID: 31900030 Free PMC article. Review.
-
γδ T Cells in Antimalarial Immunity: New Insights Into Their Diverse Functions in Protection and Tolerance.Front Immunol. 2018 Oct 23;9:2445. doi: 10.3389/fimmu.2018.02445. eCollection 2018. Front Immunol. 2018. PMID: 30405634 Free PMC article. Review.
-
Role of Vγ9vδ2 T lymphocytes in infectious diseases.Front Immunol. 2022 Jul 18;13:928441. doi: 10.3389/fimmu.2022.928441. eCollection 2022. Front Immunol. 2022. PMID: 35924233 Free PMC article. Review.
-
T cell-mediated immunity to malaria.Nat Rev Immunol. 2019 Jul;19(7):457-471. doi: 10.1038/s41577-019-0158-z. Nat Rev Immunol. 2019. PMID: 30940932 Free PMC article. Review.
References
-
- World Health Organization. World Malaria Report 2008. WHO/HTM/GMP/2008.1 2008
-
- Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. - PubMed
-
- Schofield L, Mueller I. Clinical immunity to malaria. Curr Mol Med. 2006;6:205–221. - PubMed
-
- Cockburn IA, Zavala F. T cell memory in malaria. Curr Opin Immunol. 2007;19:424–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

