Follicular dendritic cell-specific prion protein (PrP) expression alone is sufficient to sustain prion infection in the spleen
- PMID: 22144895
- PMCID: PMC3228802
- DOI: 10.1371/journal.ppat.1002402
Follicular dendritic cell-specific prion protein (PrP) expression alone is sufficient to sustain prion infection in the spleen
Abstract
Prion diseases are characterised by the accumulation of PrP(Sc), an abnormally folded isoform of the cellular prion protein (PrP(C)), in affected tissues. Following peripheral exposure high levels of prion-specific PrP(Sc) accumulate first upon follicular dendritic cells (FDC) in lymphoid tissues before spreading to the CNS. Expression of PrP(C) is mandatory for cells to sustain prion infection and FDC appear to express high levels. However, whether FDC actively replicate prions or simply acquire them from other infected cells is uncertain. In the attempts to-date to establish the role of FDC in prion pathogenesis it was not possible to dissociate the Prnp expression of FDC from that of the nervous system and all other non-haematopoietic lineages. This is important as FDC may simply acquire prions after synthesis by other infected cells. To establish the role of FDC in prion pathogenesis transgenic mice were created in which PrP(C) expression was specifically "switched on" or "off" only on FDC. We show that PrP(C)-expression only on FDC is sufficient to sustain prion replication in the spleen. Furthermore, prion replication is blocked in the spleen when PrP(C)-expression is specifically ablated only on FDC. These data definitively demonstrate that FDC are the essential sites of prion replication in lymphoid tissues. The demonstration that Prnp-ablation only on FDC blocked splenic prion accumulation without apparent consequences for FDC status represents a novel opportunity to prevent neuroinvasion by modulation of PrP(C) expression on FDC.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
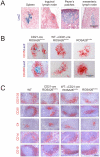

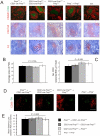

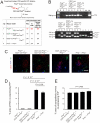
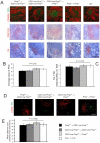
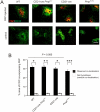


References
-
- Horiuchi M, Furuoka H, Kitamura N, Shinagawa M. Alymphoplasia mice are resistant to prion infection via oral route. Jap J Vet Res. 2006;53:149–157. - PubMed
-
- Glaysher BR, Mabbott NA. Role of the GALT in scrapie agent neuroinvasion from the intestine. J Immunol. 2007;178:3757–3766. - PubMed
-
- Andreoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, et al. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol. 2000;81:3115–3126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 083603/B/07/Z/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D00831X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/05241339/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/A/00001660/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/R/00001813/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/A/00001659/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D00831X/3/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BS516875/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D00831X/2/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00000989/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

