Genesis of mammalian prions: from non-infectious amyloid fibrils to a transmissible prion disease
- PMID: 22144901
- PMCID: PMC3228811
- DOI: 10.1371/journal.ppat.1002419
Genesis of mammalian prions: from non-infectious amyloid fibrils to a transmissible prion disease
Abstract
The transmissible agent of prion disease consists of a prion protein in its abnormal, β-sheet rich state (PrP(Sc)), which is capable of replicating itself according to the template-assisted mechanism. This mechanism postulates that the folding pattern of a newly recruited polypeptide chain accurately reproduces that of a PrP(Sc) template. Here we report that authentic PrP(Sc) and transmissible prion disease can be generated de novo in wild type animals by recombinant PrP (rPrP) amyloid fibrils, which are structurally different from PrP(Sc) and lack any detectable PrP(Sc) particles. When induced by rPrP fibrils, a long silent stage that involved two serial passages preceded development of the clinical disease. Once emerged, the prion disease was characterized by unique clinical, neuropathological, and biochemical features. The long silent stage to the disease was accompanied by significant transformation in neuropathological properties and biochemical features of the proteinase K-resistant PrP material (PrPres) before authentic PrP(Sc) evolved. The current work illustrates that transmissible prion diseases can be induced by PrP structures different from that of authentic PrP(Sc) and suggests that a new mechanism different from the classical templating exists. This new mechanism designated as "deformed templating" postulates that a change in the PrP folding pattern from the one present in rPrP fibrils to an alternative specific for PrP(Sc) can occur. The current work provides important new insight into the mechanisms underlying genesis of the transmissible protein states and has numerous implications for understanding the etiology of neurodegenerative diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
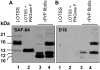
Figures



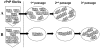
Similar articles
-
A new mechanism for transmissible prion diseases.J Neurosci. 2012 May 23;32(21):7345-55. doi: 10.1523/JNEUROSCI.6351-11.2012. J Neurosci. 2012. PMID: 22623680 Free PMC article.
-
Two alternative pathways for generating transmissible prion disease de novo.Acta Neuropathol Commun. 2015 Nov 10;3:69. doi: 10.1186/s40478-015-0248-5. Acta Neuropathol Commun. 2015. PMID: 26556038 Free PMC article.
-
Genesis of tramsmissible protein states via deformed templating.Prion. 2012 Jul 1;6(3):252-5. doi: 10.4161/pri.19930. Epub 2012 Jul 1. Prion. 2012. PMID: 22561163 Free PMC article.
-
[Mechanisms of prion transmission].Nihon Rinsho. 2007 Aug;65(8):1391-5. Nihon Rinsho. 2007. PMID: 17695274 Review. Japanese.
-
Prions.Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a006833. doi: 10.1101/cshperspect.a006833. Cold Spring Harb Perspect Biol. 2011. PMID: 21421910 Free PMC article. Review.
Cited by
-
Reversible off and on switching of prion infectivity via removing and reinstalling prion sialylation.Sci Rep. 2016 Sep 9;6:33119. doi: 10.1038/srep33119. Sci Rep. 2016. PMID: 27609323 Free PMC article.
-
Contrasting Effects of Two Lipid Cofactors of Prion Replication on the Conformation of the Prion Protein.PLoS One. 2015 Jun 19;10(6):e0130283. doi: 10.1371/journal.pone.0130283. eCollection 2015. PLoS One. 2015. PMID: 26090881 Free PMC article.
-
Heterogeneous seeding of HET-s(218-289) and the mutability of prion structures.Prion. 2014 Mar-Apr;8(2):178-82. doi: 10.4161/pri.28126. Epub 2014 Feb 18. Prion. 2014. PMID: 24549096 Free PMC article. Review.
-
Atypical and classical forms of the disease-associated state of the prion protein exhibit distinct neuronal tropism, deposition patterns, and lesion profiles.Am J Pathol. 2013 Nov;183(5):1539-1547. doi: 10.1016/j.ajpath.2013.07.024. Epub 2013 Sep 5. Am J Pathol. 2013. PMID: 24012784 Free PMC article.
-
Sialylation of the prion protein glycans controls prion replication rate and glycoform ratio.Sci Rep. 2015 Nov 18;5:16912. doi: 10.1038/srep16912. Sci Rep. 2015. PMID: 26576925 Free PMC article.
References
-
- Prusiner SB. Prion diseases. In: Nathanson N, Ahmed R, Gonzalez-Scarano F, Griffin D, Holmes K, et al., editors. Viral Pathogenesis. New York: Raven Press; 1996. pp. 855–911.
-
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. - PubMed
-
- Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–1044. - PubMed
-
- Legname G, Baskakov IV, Nguyen H-OB, Riesner D, Cohen FE, et al. Synthetic mammalian prions. Science. 2004;305:673–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

