Genome instability and transcription elongation impairment in human cells depleted of THO/TREX
- PMID: 22144908
- PMCID: PMC3228816
- DOI: 10.1371/journal.pgen.1002386
Genome instability and transcription elongation impairment in human cells depleted of THO/TREX
Abstract
THO/TREX connects transcription with genome integrity in yeast, but a role of mammalian THO in these processes is uncertain, which suggests a differential implication of mRNP biogenesis factors in genome integrity in yeast and humans. We show that human THO depletion impairs transcription elongation and mRNA export and increases instability associated with DNA breaks, leading to hyper-recombination and γH2AX and 53BP1 foci accumulation. This is accompanied by replication alteration as determined by DNA combing. Genome instability is R-loop-dependent, as deduced from the ability of the AID enzyme to increase DNA damage and of RNaseH to reduce it, or from the enhancement of R-loop-dependent class-switching caused by THOC1-depletion in CH12 murine cells. Therefore, mammalian THO prevents R-loop formation and has a role in genome dynamics and function consistent with an evolutionary conservation of the functional connection between these mRNP biogenesis factors and genome integrity that had not been anticipated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
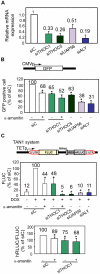
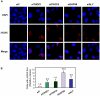

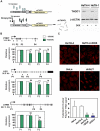
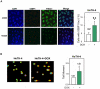
Similar articles
-
The interface between transcription and mRNP export: from THO to THSC/TREX-2.Biochim Biophys Acta. 2010 Aug;1799(8):533-8. doi: 10.1016/j.bbagrm.2010.06.002. Epub 2010 Jun 19. Biochim Biophys Acta. 2010. PMID: 20601280 Review.
-
BRCA2 prevents R-loop accumulation and associates with TREX-2 mRNA export factor PCID2.Nature. 2014 Jul 17;511(7509):362-5. doi: 10.1038/nature13374. Epub 2014 Jun 1. Nature. 2014. PMID: 24896180
-
The THP1-SAC3-SUS1-CDC31 complex works in transcription elongation-mRNA export preventing RNA-mediated genome instability.Mol Biol Cell. 2008 Oct;19(10):4310-8. doi: 10.1091/mbc.e08-04-0355. Epub 2008 Jul 30. Mol Biol Cell. 2008. PMID: 18667528 Free PMC article.
-
Human THO-Sin3A interaction reveals new mechanisms to prevent R-loops that cause genome instability.EMBO J. 2017 Dec 1;36(23):3532-3547. doi: 10.15252/embj.201797208. Epub 2017 Oct 26. EMBO J. 2017. PMID: 29074626 Free PMC article.
-
New clues to understand the role of THO and other functionally related factors in mRNP biogenesis.Biochim Biophys Acta. 2012 Jun;1819(6):514-20. doi: 10.1016/j.bbagrm.2011.11.012. Epub 2011 Dec 20. Biochim Biophys Acta. 2012. PMID: 22207203 Review.
Cited by
-
Pre-mRNA processing factors meet the DNA damage response.Front Genet. 2013 Jun 6;4:102. doi: 10.3389/fgene.2013.00102. eCollection 2013. Front Genet. 2013. PMID: 23761808 Free PMC article.
-
Comprehensive Analysis of the Expression and Clinical Significance of THO Complex Members in Hepatocellular Carcinoma.Int J Gen Med. 2022 Mar 8;15:2695-2713. doi: 10.2147/IJGM.S349925. eCollection 2022. Int J Gen Med. 2022. PMID: 35300138 Free PMC article.
-
Elevated level of PLRG1 is critical for the proliferation and maintenance of genome stability of tumor cells.BMB Rep. 2023 Nov;56(11):612-617. doi: 10.5483/BMBRep.2023-0162. BMB Rep. 2023. PMID: 37817442 Free PMC article.
-
DICER ribonuclease removes harmful R-loops.Mol Cell. 2023 Oct 19;83(20):3707-3719.e5. doi: 10.1016/j.molcel.2023.09.021. Epub 2023 Oct 11. Mol Cell. 2023. PMID: 37827159 Free PMC article.
-
The chromatin network helps prevent cancer-associated mutagenesis at transcription-replication conflicts.Nat Commun. 2023 Oct 28;14(1):6890. doi: 10.1038/s41467-023-42653-0. Nat Commun. 2023. PMID: 37898641 Free PMC article.
References
-
- Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr Opin Cell Biol. 2005;17:251–256. - PubMed
-
- Luna R, Gaillard H, Gonzalez-Aguilera C, Aguilera A. Biogenesis of mRNPs: integrating different processes in the eukaryotic nucleus. Chromosoma. 2008;117:319–331. - PubMed
-
- Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417:304–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

