The fission yeast stress-responsive MAPK pathway promotes meiosis via the phosphorylation of Pol II CTD in response to environmental and feedback cues
- PMID: 22144909
- PMCID: PMC3228818
- DOI: 10.1371/journal.pgen.1002387
The fission yeast stress-responsive MAPK pathway promotes meiosis via the phosphorylation of Pol II CTD in response to environmental and feedback cues
Abstract
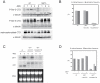
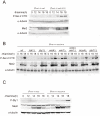
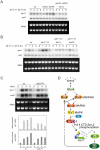
The RRM-type RNA-binding protein Mei2 is a master regulator of meiosis in fission yeast, in which it stabilizes meiosis-specific mRNAs by blocking their destruction. Artificial activation of Mei2 can provoke the entire meiotic process, and it is suspected that Mei2 may do more than the stabilization of meiosis-specific mRNAs. In our current study using a new screening system, we show that Mei2 genetically interacts with subunits of CTDK-I, which phosphorylates serine-2 residues on the C-terminal domain of RNA polymerase II (Pol II CTD). Phosphorylation of CTD Ser-2 is essential to enable the robust transcription of ste11, which encodes an HMG-type transcription factor that regulates the expression of mei2 and other genes necessary for sexual development. CTD Ser-2 phosphorylation increases under nitrogen starvation, and the stress-responsive MAP kinase pathway, mediated by Wis1 MAPKK and Sty1 MAPK, is critical for this stress response. Sty1 phosphorylates Lsk1, the catalytic subunit of CTDK-I. Furthermore, a feedback loop stemming from activated Mei2 to Win1 and Wis4 MAPKKKs operates in this pathway and eventually enhances CTD Ser-2 phosphorylation and ste11 transcription. Hence, in addition to starting meiosis, Mei2 functions to reinforce the commitment to it, once cells have entered this process. This study also demonstrates clearly that the stress-responsive MAP kinase pathway can modulates gene expression through phosphorylation of Pol II CTD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast.Genes Dev. 1996 Sep 15;10(18):2289-301. doi: 10.1101/gad.10.18.2289. Genes Dev. 1996. PMID: 8824588
-
Constitutive activation of the fission yeast pheromone-responsive pathway induces ectopic meiosis and reveals ste11 as a mitogen-activated protein kinase target.Mol Cell Biol. 2005 Mar;25(5):2045-59. doi: 10.1128/MCB.25.5.2045-2059.2005. Mol Cell Biol. 2005. PMID: 15713656 Free PMC article.
-
The fission yeast Pin1 peptidyl-prolyl isomerase promotes dissociation of Sty1 MAPK from RNA polymerase II and recruits Ssu72 phosphatase to facilitate oxidative stress induced transcription.Nucleic Acids Res. 2021 Jan 25;49(2):805-817. doi: 10.1093/nar/gkaa1243. Nucleic Acids Res. 2021. PMID: 33410907 Free PMC article.
-
Regulation of meiosis in fission yeast.Cell Struct Funct. 1996 Oct;21(5):431-6. doi: 10.1247/csf.21.431. Cell Struct Funct. 1996. PMID: 9118252 Review.
-
The selective elimination of messenger RNA underlies the mitosis-meiosis switch in fission yeast.Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(8):788-97. doi: 10.2183/pjab.86.788. Proc Jpn Acad Ser B Phys Biol Sci. 2010. PMID: 20948174 Free PMC article. Review.
Cited by
-
Distinct requirement of RNA polymerase II CTD phosphorylations in budding and fission yeast.Transcription. 2012 Sep-Oct;3(5):231-4. doi: 10.4161/trns.21066. Epub 2012 Sep 1. Transcription. 2012. PMID: 22771993 Free PMC article.
-
Rhn1, a nuclear protein, is required for suppression of meiotic mRNAs in mitotically dividing fission yeast.PLoS One. 2012;7(8):e42962. doi: 10.1371/journal.pone.0042962. Epub 2012 Aug 17. PLoS One. 2012. PMID: 22912768 Free PMC article.
-
Modulation of RNA polymerase II phosphorylation downstream of pathogen perception orchestrates plant immunity.Cell Host Microbe. 2014 Dec 10;16(6):748-58. doi: 10.1016/j.chom.2014.10.018. Epub 2014 Nov 26. Cell Host Microbe. 2014. PMID: 25464831 Free PMC article.
-
Role of the fission yeast cell integrity MAPK pathway in response to glucose limitation.BMC Microbiol. 2013 Feb 11;13:34. doi: 10.1186/1471-2180-13-34. BMC Microbiol. 2013. PMID: 23398982 Free PMC article.
-
The SrkA Kinase Is Part of the SakA Mitogen-Activated Protein Kinase Interactome and Regulates Stress Responses and Development in Aspergillus nidulans.Eukaryot Cell. 2015 May;14(5):495-510. doi: 10.1128/EC.00277-14. Epub 2015 Mar 27. Eukaryot Cell. 2015. PMID: 25820520 Free PMC article.
References
-
- Mata J, Lyne R, Burns G, Bahler J. The transcriptional program of meiosis and sporulation in fission yeast. Nat Genet. 2002;32:143–147. - PubMed
-
- Kassir Y, Adir N, Boger-Nadjar E, Raviv NG, Rubin-Bejerano I, et al. Transcriptional regulation of meiosis in budding yeast. Int Rev Cytol. 2003;224:111–171. - PubMed
-
- Harigaya Y, Tanaka H, Yamanaka S, Tanaka K, Watanabe Y, et al. Selective elimination of messenger RNA prevents an incidence of untimely meiosis. Nature. 2006;442:45–50. - PubMed
-
- Watanabe Y, Yamamoto M. S.pombe mei2 + encodes an RNA-binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species meiRNA. Cell. 1994;78:487–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

