Targeted proteolysis of plectin isoform 1a accounts for hemidesmosome dysfunction in mice mimicking the dominant skin blistering disease EBS-Ogna
- PMID: 22144912
- PMCID: PMC3228830
- DOI: 10.1371/journal.pgen.1002396
Targeted proteolysis of plectin isoform 1a accounts for hemidesmosome dysfunction in mice mimicking the dominant skin blistering disease EBS-Ogna
Abstract
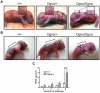
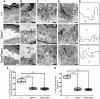
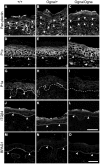
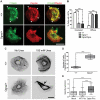
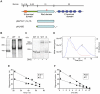
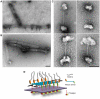
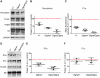
Autosomal recessive mutations in the cytolinker protein plectin account for the multisystem disorders epidermolysis bullosa simplex (EBS) associated with muscular dystrophy (EBS-MD), pyloric atresia (EBS-PA), and congenital myasthenia (EBS-CMS). In contrast, a dominant missense mutation leads to the disease EBS-Ogna, manifesting exclusively as skin fragility. We have exploited this trait to study the molecular basis of hemidesmosome failure in EBS-Ogna and to reveal the contribution of plectin to hemidesmosome homeostasis. We generated EBS-Ogna knock-in mice mimicking the human phenotype and show that blistering reflects insufficient protein levels of the hemidesmosome-associated plectin isoform 1a. We found that plectin 1a, in contrast to plectin 1c, the major isoform expressed in epidermal keratinocytes, is proteolytically degraded, supporting the notion that degradation of hemidesmosome-anchored plectin is spatially controlled. Using recombinant proteins, we show that the mutation renders plectin's 190-nm-long coiled-coil rod domain more vulnerable to cleavage by calpains and other proteases activated in the epidermis but not in skeletal muscle. Accordingly, treatment of cultured EBS-Ogna keratinocytes as well as of EBS-Ogna mouse skin with calpain inhibitors resulted in increased plectin 1a protein expression levels. Moreover, we report that plectin's rod domain forms dimeric structures that can further associate laterally into remarkably stable (paracrystalline) polymers. We propose focal self-association of plectin molecules as a novel mechanism contributing to hemidesmosome homeostasis and stabilization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

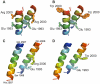

Similar articles
-
Mutation in exon 1a of PLEC, leading to disruption of plectin isoform 1a, causes autosomal-recessive skin-only epidermolysis bullosa simplex.Hum Mol Genet. 2015 Jun 1;24(11):3155-62. doi: 10.1093/hmg/ddv066. Epub 2015 Feb 24. Hum Mol Genet. 2015. PMID: 25712130
-
A site-specific plectin mutation causes dominant epidermolysis bullosa simplex Ogna: two identical de novo mutations.J Invest Dermatol. 2002 Jan;118(1):87-93. doi: 10.1046/j.0022-202x.2001.01591.x. J Invest Dermatol. 2002. PMID: 11851880
-
The many faces of plectin and plectinopathies: pathology and mechanisms.Acta Neuropathol. 2013 Jan;125(1):77-93. doi: 10.1007/s00401-012-1026-0. Epub 2012 Aug 3. Acta Neuropathol. 2013. PMID: 22864774 Review.
-
Plectin-related skin diseases.J Dermatol Sci. 2015 Mar;77(3):139-45. doi: 10.1016/j.jdermsci.2014.11.005. Epub 2014 Nov 28. J Dermatol Sci. 2015. PMID: 25530118 Review.
-
Plectin deficiency leads to both muscular dystrophy and pyloric atresia in epidermolysis bullosa simplex.Hum Mutat. 2010 Oct;31(10):E1687-98. doi: 10.1002/humu.21330. Hum Mutat. 2010. PMID: 20665883 Free PMC article.
Cited by
-
Properties of intermediate filament networks assembled from keratin 8 and 18 in the presence of Mg²+.Biophys J. 2012 Jul 18;103(2):195-201. doi: 10.1016/j.bpj.2012.06.014. Epub 2012 Jul 17. Biophys J. 2012. PMID: 22853896 Free PMC article.
-
The rod domain is not essential for the function of plectin in maintaining tissue integrity.Mol Biol Cell. 2015 Jul 1;26(13):2402-17. doi: 10.1091/mbc.E15-01-0043. Epub 2015 May 13. Mol Biol Cell. 2015. PMID: 25971800 Free PMC article.
-
Plectin-Mediated Intermediate Filament Functions: Why Isoforms Matter.Cells. 2021 Aug 21;10(8):2154. doi: 10.3390/cells10082154. Cells. 2021. PMID: 34440923 Free PMC article. Review.
-
Plectin in Skin Fragility Disorders.Cells. 2021 Oct 14;10(10):2738. doi: 10.3390/cells10102738. Cells. 2021. PMID: 34685719 Free PMC article. Review.
-
Vimentin Intermediate Filaments as Potential Target for Cancer Treatment.Cancers (Basel). 2020 Jan 11;12(1):184. doi: 10.3390/cancers12010184. Cancers (Basel). 2020. PMID: 31940801 Free PMC article. Review.
References
-
- Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383. - PubMed
-
- Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci. 1998;111(Pt 17):2477–2486. - PubMed
-
- Rezniczek GA, Walko G, Wiche G. Plectin gene defects lead to various forms of epidermolysis bullosa simplex. Dermatol Clin. 2010;28:33–41. - PubMed
-
- Rezniczek GA, Abrahamsberg C, Fuchs P, Spazierer D, Wiche G. Plectin 5′-transcript diversity: short alternative sequences determine stability of gene products, initiation of translation and subcellular localization of isoforms. Hum Mol Genet. 2003;12:3181–3194. - PubMed
-
- Fuchs P, Zorer M, Rezniczek GA, Spazierer D, Oehler S, et al. Unusual 5′ transcript complexity of plectin isoforms: novel tissue-specific exons modulate actin binding activity. Hum Mol Genet. 1999;8:2461–2472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

