Neural degeneration in the retina of the streptozotocin-induced type 1 diabetes model
- PMID: 22144984
- PMCID: PMC3226536
- DOI: 10.1155/2011/108328
Neural degeneration in the retina of the streptozotocin-induced type 1 diabetes model
Abstract
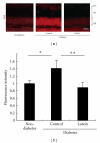
Diabetic retinopathy, a vision-threatening disease, has been regarded as a vascular disorder. However, impaired oscillatory potentials (OPs) in the electroretinogram (ERG) and visual dysfunction are recorded before severe vascular lesions appear. Here, we review the molecular mechanisms underlying the retinal neural degeneration observed in the streptozotocin-(STZ-) induced type 1 diabetes model. The renin-angiotensin system (RAS) and reactive oxygen species (ROS) both cause OP impairment and reduced levels of synaptophysin, a synaptic vesicle protein for neurotransmitter release, most likely through excessive protein degradation by the ubiquitin-proteasome system. ROS also decrease brain-derived neurotrophic factor (BDNF) and inner retinal neuronal cells. The influence of both RAS and ROS on synaptophysin suggests that RAS-ROS crosstalk occurs in the diabetic retina. Therefore, suppressors of RAS or ROS, such as angiotensin II type 1 receptor blockers or the antioxidant lutein, respectively, are potential candidates for neuroprotective and preventive therapies to improve the visual prognosis.
Copyright © 2011 Yoko Ozawa et al.
Figures


Similar articles
-
Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes.Diabetologia. 2010 May;53(5):971-9. doi: 10.1007/s00125-009-1655-6. Epub 2010 Feb 17. Diabetologia. 2010. PMID: 20162412 Free PMC article.
-
Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina.Diabetes. 2008 Aug;57(8):2191-8. doi: 10.2337/db07-1281. Epub 2008 May 16. Diabetes. 2008. PMID: 18487452 Free PMC article.
-
PRMT-1 and DDAHs-induced ADMA upregulation is involved in ROS- and RAS-mediated diabetic retinopathy.Exp Eye Res. 2009 Dec;89(6):1028-34. doi: 10.1016/j.exer.2009.09.004. Epub 2009 Sep 11. Exp Eye Res. 2009. PMID: 19748504
-
Angiotensin and diabetic retinopathy.Int J Biochem Cell Biol. 2006;38(5-6):752-65. doi: 10.1016/j.biocel.2005.08.002. Epub 2005 Sep 1. Int J Biochem Cell Biol. 2006. PMID: 16165393 Review.
-
Role of Tissue Renin-angiotensin System and the Chymase/angiotensin-( 1-12) Axis in the Pathogenesis of Diabetic Retinopathy.Curr Med Chem. 2017;24(28):3104-3114. doi: 10.2174/0929867324666170407141955. Curr Med Chem. 2017. PMID: 28403787 Free PMC article. Review.
Cited by
-
The role of tumor necrosis factor-α and interferon-γ in the hyperglycemia-induced ubiquitination and loss of platelet endothelial cell adhesion molecule-1 in rat retinal endothelial cells.Microcirculation. 2021 Oct;28(7):e12717. doi: 10.1111/micc.12717. Epub 2021 May 29. Microcirculation. 2021. PMID: 34008903 Free PMC article.
-
Retinal and circulating miRNA expression patterns in diabetic retinopathy: An in silico and in vivo approach.Br J Pharmacol. 2019 Jul;176(13):2179-2194. doi: 10.1111/bph.14665. Epub 2019 May 9. Br J Pharmacol. 2019. PMID: 30883703 Free PMC article.
-
Influence of vanadium-organic ligands treatment on selected metal levels in kidneys of STZ rats.Biol Trace Elem Res. 2013 Jun;153(1-3):319-28. doi: 10.1007/s12011-013-9688-6. Epub 2013 May 11. Biol Trace Elem Res. 2013. PMID: 23661329 Free PMC article.
-
Oxidative Stress and Vascular Dysfunction in the Retina: Therapeutic Strategies.Antioxidants (Basel). 2020 Aug 17;9(8):761. doi: 10.3390/antiox9080761. Antioxidants (Basel). 2020. PMID: 32824523 Free PMC article. Review.
-
Ubiquitin-proteasome system in diabetic retinopathy.PeerJ. 2022 Jul 19;10:e13715. doi: 10.7717/peerj.13715. eCollection 2022. PeerJ. 2022. PMID: 35873915 Free PMC article. Review.
References
-
- Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D. Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Japanese Journal of Ophthalmology. 2006;50(4):367–373. - PubMed
-
- Yonemura D, Aoki T, Tsuzuki K. Electroretinogram in diabetic retinopathy. Archives of Ophthalmology. 1962;68:19–24. - PubMed
-
- Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity: specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43(11):1326–1333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
