Involvement of F-actin in chaperonin-containing t-complex 1 beta regulating mouse mesangial cell functions in a glucose-induction cell model
- PMID: 22144988
- PMCID: PMC3227506
- DOI: 10.1155/2011/565647
Involvement of F-actin in chaperonin-containing t-complex 1 beta regulating mouse mesangial cell functions in a glucose-induction cell model
Abstract
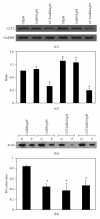
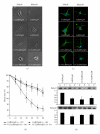
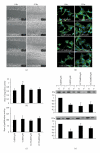
The aim of this study is to investigate the role of chaperonin-containing t-complex polypeptide 1 beta (CCT2) in the regulation of mouse mesangial cell (mMC) contraction, proliferation, and migration with filamentous/globular-(F/G-) actin ratio under high glucose induction. A low CCT2 mMC model induced by treatment of small interference RNA was established. Groups with and without low CCT2 induction examined in normal and high (H) glucose conditions revealed the following major results: (1) low CCT2 or H glucose showed the ability to attenuate F/G-actin ratio; (2) groups with low F/G-actin ratio all showed less cell contraction; (3) suppression of CCT2 may reduce the proliferation and migration which were originally induced by H glucose. In conclusion, CCT2 can be used as a specific regulator for mMC contraction, proliferation, and migration affected by glucose, which mechanism may involve the alteration of F-actin, particularly for cell contraction.
Copyright © 2011 Jin-Shuen Chen et al.
Figures

References
-
- Ueta M, Wakisaka M, Ago T, et al. PPARγ ligands attenuate mesangial contractile dysfunction in high glucose. Kidney International. 2004;65(3):961–971. - PubMed
-
- Cove-Smith A, Hendry BM. The regulation of mesangial cell proliferation. Nephron—Experimental Nephrology. 2008;108(4):e74–e79. - PubMed
-
- Schlöndorff D, Banas B. The mesangial cell revisited: no cell is an island. Journal of the American Society of Nephrology. 2009;20(6):1179–1187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
