TRAIL and DcR1 expressions are differentially regulated in the pancreatic islets of STZ- versus CY-applied NOD mice
- PMID: 22144989
- PMCID: PMC3226359
- DOI: 10.1155/2011/625813
TRAIL and DcR1 expressions are differentially regulated in the pancreatic islets of STZ- versus CY-applied NOD mice
Abstract
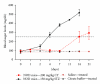
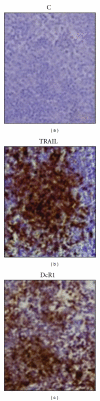
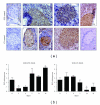
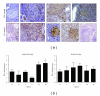
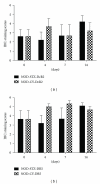
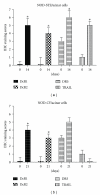
TNF-related apoptosis-inducing ligand (TRAIL) is an important component of the immune system. Although it is well acknowledged that it also has an important role in Type 1 Diabetes (T1D) development, this presumed role has not yet been clearly revealed. Streptozotocin (STZ) and Cyclophosphamide (CY) are frequently used agents for establishment or acceleration of T1D disease in experimental models, including the non-obese diabetic (NOD) mice. Although such disease models are very suitable for diabetes research, different expression patterns for various T1D-related molecules may be expected, depending on the action mechanism of the applied agent. We accelerated diabetes in female NOD mice using STZ or CY and analyzed the expression profiles of TRAIL ligand and receptors throughout disease development. TRAIL ligand expression followed a completely different pattern in STZ- versus CY-accelerated disease, displaying a prominent increase in the former, while appearing at reduced levels in the latter. Decoy receptor 1 (DcR1) expression also increased significantly in the pancreatic islets in STZ-induced disease. Specific increases observed in TRAIL ligand and DcR1 expressions may be part of a defensive strategy of the beta islets against the infiltrating leukocytes, while the immune-suppressive agent CY may partly hold down this defense, contributing further to diabetes development.
Copyright © 2011 Ercument Dirice et al.
Figures

Similar articles
-
Nicotinamide prevents the development of diabetes in the cyclophosphamide-induced NOD mouse model by reducing beta-cell apoptosis.J Pathol. 2000 May;191(1):86-92. doi: 10.1002/(SICI)1096-9896(200005)191:1<86::AID-PATH573>3.0.CO;2-0. J Pathol. 2000. PMID: 10767724
-
Blockade of tumor necrosis factor-related apoptosis-inducing ligand exacerbates type 1 diabetes in NOD mice.Diabetes. 2003 Aug;52(8):1967-75. doi: 10.2337/diabetes.52.8.1967. Diabetes. 2003. PMID: 12882912
-
Fas and Fas ligand immunolocalization in pancreatic islets of NOD mice during spontaneous and cyclophosphamide-accelerated diabetes.Histochem J. 2002 Jan-Feb;34(1-2):1-12. doi: 10.1023/a:1021321522826. Histochem J. 2002. PMID: 12365794
-
Immunomodulatory Functions of TNF-Related Apoptosis-Inducing Ligand in Type 1 Diabetes.Cells. 2024 Oct 10;13(20):1676. doi: 10.3390/cells13201676. Cells. 2024. PMID: 39451194 Free PMC article. Review.
-
Repositioning the Role of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) on the TRAIL to the Development of Diabetes Mellitus: An Update of Experimental and Clinical Evidence.Int J Mol Sci. 2022 Mar 17;23(6):3225. doi: 10.3390/ijms23063225. Int J Mol Sci. 2022. PMID: 35328646 Free PMC article. Review.
Cited by
-
The double trouble of metabolic diseases: the diabetes-cancer link.Mol Biol Cell. 2015 Sep 15;26(18):3129-39. doi: 10.1091/mbc.E14-11-1550. Mol Biol Cell. 2015. PMID: 26371080 Free PMC article.
-
Tolerogenic vaccine composited with islet-derived multipeptides and cyclosporin A induces pTreg and prevents Type 1 diabetes in murine model.Hum Vaccin Immunother. 2020;16(2):240-250. doi: 10.1080/21645515.2019.1616504. Epub 2019 Oct 23. Hum Vaccin Immunother. 2020. PMID: 31070990 Free PMC article.
-
Association of circulating sTRAIL and high-sensitivity CRP with type 2 diabetic nephropathy and foot ulcers.Med Sci Monit. 2013 Aug 29;19:712-5. doi: 10.12659/MSM.889514. Med Sci Monit. 2013. PMID: 23986130 Free PMC article.
-
Lentiviral gene therapy vectors encoding VIP suppressed diabetes-related inflammation and augmented pancreatic beta-cell proliferation.Gene Ther. 2021 Apr;28(3-4):130-141. doi: 10.1038/s41434-020-0183-3. Epub 2020 Jul 30. Gene Ther. 2021. PMID: 32733091
-
Elevated Serum Osteoprotegerin is Associated with Reduced Risks of Albuminuria and CKD Progression in Patients with Type 2 Diabetes.Diabetes Metab Syndr Obes. 2022 Dec 12;15:3831-3841. doi: 10.2147/DMSO.S390483. eCollection 2022. Diabetes Metab Syndr Obes. 2022. PMID: 36530588 Free PMC article.
References
-
- Atkinson MA, Maclaren NK. Mechanisms of disease: the pathogenesis of insulin-dependent diabetes mellitus. New England Journal of Medicine. 1994;331(21):1428–1436. - PubMed
-
- Thomas HE, Kay TWH. Beta cell destruction in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Diabetes/Metabolism Research and Reviews. 2000;16(4):251–261. - PubMed
-
- Lamhamedi-Cherradi SE, Zheng S, Tisch RM, Chen YH. Critical roles of tumor necrosis factor-related apoptosis-inducing ligand in type 1 diabetes. Diabetes. 2003;52(9):2274–2278. - PubMed
-
- Cheung SSC, Metzger DL, Wang X, et al. Tumor necrosis factor-related apoptosis-inducing ligand and CD56 expression in patients with type 1 diabetes mellitus. Pancreas. 2005;30(2):105–114. - PubMed
-
- Mi QS, Ly D, Lamhamedi-Cherradi SE, et al. Blockade of tumor necrosis factor-related apoptosis-inducing ligand exacerbates type 1 diabetes in NOD mice. Diabetes. 2003;52(8):1967–1975. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
