Identification of major degradation products of ketoconazole
- PMID: 22145107
- PMCID: PMC3221500
- DOI: 10.3797/scipharm.1107-18
Identification of major degradation products of ketoconazole
Abstract
Analytical methods were developed for the identification of major degradation products of Ketoconazole, an antifungal agent. The stressed degradation of Ketoconazole drug substance was performed under acid, base, thermal, photo and oxidative stress conditions. The major degradation was observed under acid, base and oxidative stress conditions. The degradation study was performed on Inertsil ODS-3V, length 100 X diameter 4.6 mm, particle size 3 μm column using gradient method. These degradants were identified by LC-MS technique.
Keywords: Hydrolysis degradent; Ketoconazole; LC-MS; Oxidative degradent; Stress degradation.
Figures
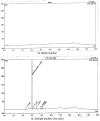




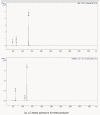






References
-
- Vander Heyde Y, Nguyet AN, Detaevenier MR, Massart DL, Plaizier-Vercammen J. Simultaneous determination of ketoconazole and formaldehyde in a shampoo: liquid chromatography method development and validation. J Chromatogr A. 2002;958:191–201. http://www.ncbi.nlm.nih.gov/pubmed/12134817. - PubMed
-
- Nguyen MN, Tallieu L, Plaizier-Vercammen J, Massart DL, Vander Heyden Y. Validation of an HPLC method on short columns to assay ketoconazole and formaldehyde in shampoo. J Pharm Biomed Anal. 2003;32:1–19. http://dx.doi.org/10.1016/S0731-7085(02)00640-4. - DOI - PubMed
-
- Abdel-Moety EM, Khattab FI, Kelani KM, AbouAl-Alamein AM. Chromatographic determination of clotrimazole, ketoconazole and fluconazole in pharmaceutical formulations. Farmaco. 2002;57:931–938. http://dx.doi.org/10.1016/S0014-827X(02)01270-3. - DOI - PubMed
-
- Vertzoni MV, Reppas C, Archontaki HA. Optimization and validation of a high-performance liquid chromatographic method with UV detection for the determination of ketoconazole in canine plasma. J Chromatogr B. 2006;839:62–67. http://dx.doi.org/10.1016/j.jchromb.2006.03.010. - DOI - PubMed
-
- Velikinac I, Cudina O, Janković I, Agbaba D, Vladimirov S. Comparison of capillary zone electrophoresis and high performance liquid chromatography methods for quantitative determination of ketoconazole in drug formulations. Farmaco. 2004;59:419–424. http://dx.doi.org/10.1016/j.farmac.2003.11.019. - DOI - PubMed
LinkOut - more resources
Full Text Sources
