Downregulating hypoxia-inducible factor-2α improves the efficacy of doxorubicin in the treatment of hepatocellular carcinoma
- PMID: 22145922
- PMCID: PMC7712417
- DOI: 10.1111/j.1349-7006.2011.02177.x
Downregulating hypoxia-inducible factor-2α improves the efficacy of doxorubicin in the treatment of hepatocellular carcinoma
Abstract
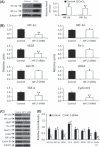
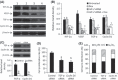
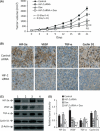
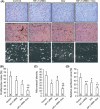
The hypoxic microenvironment inside solid tumors, including hepatocellular carcinoma (HCC), is a major cause of tumor resistance to chemotherapy. The recently identified hypoxia-inducible factor (HIF)-2 executes the hypoxia response. Its expression feature and transcriptional targets indicate a possible dominance of HIF-2 in regulating genes in HCC. The aim of the present study was to determine whether transfection of siRNA targeting HIF-2α could enhance the efficacy of doxorubicin, the most commonly used drug in the treatment of HCC. Transfection of HIF-2 siRNA into human HCC cells downregulated the expression of HIF-2α, vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-α, and cyclin D1, but had little effect on the expression of HIF-1α, fms-related tyrosine kinase-1 (Flt-1), the glucose transporter (GLUT)-1, and lactate dehydrogenase A (LDHA). Doxorubicin itself only downregulated VEGF expression. Furthermore, HIF-2 siRNA inhibited proliferation, induced cell cycle arrest at the G(0)/G(1) phase, and acted synergistically with doxorubicin to inhibit the growth of human HCC cells in vitro. Transfection of HIF-2 siRNA also downregulated tumoral expression of HIF-2α, VEGF, TGF-α, and cyclin D1 in vivo, and acted synergistically with doxorubicin to suppress the growth of HepG2 tumors established in immunodeficient mice by inhibiting cell proliferation, tumor angiogenesis and microvessel perfusion. The results of the present study suggest that targeting HIF-2α with siRNA warrants investigation as a potential strategy to enhance the efficacy of doxorubicin in the treatment of HCC.
© 2011 Japanese Cancer Association.
Figures


References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. - PubMed
-
- Gish RG, Porta C, Lazar L et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol 2007; 25: 3069–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

