Carbon monoxide, generated by heme oxygenase-1, mediates the enhanced permeability and retention effect in solid tumors
- PMID: 22145952
- PMCID: PMC7713620
- DOI: 10.1111/j.1349-7006.2011.02178.x
Carbon monoxide, generated by heme oxygenase-1, mediates the enhanced permeability and retention effect in solid tumors
Abstract
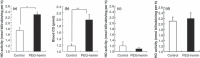
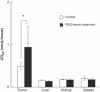
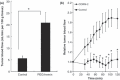
The enhanced permeability and retention (EPR) effect is a unique pathophysiological phenomenon of solid tumors that sees biocompatible macromolecules (>40 kDa) accumulate selectively in the tumor. Various factors have been implicated in this effect. Herein, we report that heme oxygenase-1 (HO-1; also known as heat shock protein 32) significantly increases vascular permeability and thus macromolecular drug accumulation in tumors. Intradermal injection of recombinant HO-1 in mice, followed by i.v. administration of a macromolecular Evans blue-albumin complex, resulted in dose-dependent extravasation of Evans blue-albumin at the HO-1 injection site. Almost no extravasation was detected when inactivated HO-1 or a carbon monoxide (CO) scavenger was injected instead. Because HO-1 generates CO, these data imply that CO plays a key role in vascular leakage. This is supported by results obtained after intratumoral administration of a CO-releasing agent (tricarbonyldichlororuthenium(II) dimer) in the same experimental setting, specifically dose-dependent increases in vascular permeability plus augmented tumor blood flow. In addition, induction of HO-1 in tumors by the water-soluble macromolecular HO-1 inducer pegylated hemin significantly increased tumor blood flow and Evans blue-albumin accumulation in tumors. These findings suggest that HO-1 and/or CO are important mediators of the EPR effect. Thus, anticancer chemotherapy using macromolecular drugs may be improved by combination with an HO-1 inducer, such as pegylated hemin, via an enhanced EPR effect.
© 2011 Japanese Cancer Association.
Figures



References
-
- Hassett MJ, O’Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy‐related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst 2006; 98: 1108–17. - PubMed
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res 1986; 46: 6387–92. - PubMed
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–6. - PubMed
-
- Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992; 267: 10931–4. - PubMed
-
- Maeda H, Matsumura Y, Kato H. Purification and identification of [hydroxyprolyl3]bradykinin in ascitic fluid from a patient with gastric cancer. J Biol Chem 1988; 263: 16051–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

