A Phase I, dose-finding and pharmacokinetic study of olaparib (AZD2281) in Japanese patients with advanced solid tumors
- PMID: 22145984
- PMCID: PMC7713608
- DOI: 10.1111/j.1349-7006.2011.02179.x
A Phase I, dose-finding and pharmacokinetic study of olaparib (AZD2281) in Japanese patients with advanced solid tumors
Abstract
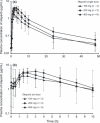
Olaparib (AZD2281) is an orally active Poly(ADP-ribose) polymerase (PARP) inhibitor with favorable antitumor activity in advanced ovarian and breast cancers with BRCA1/2 mutations in Western (USA and European) studies. This Phase I dose-finding study evaluated the tolerability, pharmacokinetics, PARP inhibitory activity, and antitumor activity of olaparib in Japanese patients with solid tumors. Olaparib was administered as a single-dose on day 1, followed by twice-daily dosing for 28 days from 48 h after a single dose. Doses were escalated from 100 mg b.i.d. in successive cohorts, up to a maximum of 400 mg b.i.d. The present study enrolled 12 patients (n = 3, 3, and 6 in 100, 200 and 400-mg b.i.d. levels, respectively). The most common adverse events were nausea, increased blood creatinine, decreased hematocrit, leukopenia and lymphopenia; dose-limiting toxicities were not observed up to and including the 400-mg b.i.d. dose level. Following twice-daily dosing, olaparib showed no marked increase in exposure at steady state over that expected from the single-dose pharmacokinetics. PARP-1 inhibition was observed from the 100-mg b.i.d. dose level in peripheral blood mononuclear cells from 6 h post-dose on day 1 during the multiple-dosing period. A patient with metastatic breast cancer (100 mg b.i.d.) had a partial response for 13 months and four patients (two each in the 200 and 400-mg b.i.d. levels) had stable disease >8 weeks. Olaparib was well tolerated up to the 400-mg b.i.d. dose in Japanese patients with solid tumors. Preliminary evidence of antitumor activity was observed.
Trial registration: ClinicalTrials.gov NCT00572364.
© 2011 Japanese Cancer Association.
Figures
Similar articles
-
Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study.Invest New Drugs. 2012 Aug;30(4):1493-500. doi: 10.1007/s10637-011-9682-9. Epub 2011 May 18. Invest New Drugs. 2012. PMID: 21590367 Clinical Trial.
-
Safety and tolerability of the olaparib tablet formulation in Japanese patients with advanced solid tumours.Cancer Chemother Pharmacol. 2016 Sep;78(3):525-31. doi: 10.1007/s00280-016-3106-7. Epub 2016 Jul 15. Cancer Chemother Pharmacol. 2016. PMID: 27422301 Free PMC article. Clinical Trial.
-
A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer.Eur J Cancer. 2013 Sep;49(14):2972-8. doi: 10.1016/j.ejca.2013.05.020. Epub 2013 Jun 27. Eur J Cancer. 2013. PMID: 23810467 Free PMC article. Clinical Trial.
-
Cediranib, a pan-VEGFR inhibitor, and olaparib, a PARP inhibitor, in combination therapy for high grade serous ovarian cancer.Expert Opin Investig Drugs. 2016;25(5):597-611. doi: 10.1517/13543784.2016.1156857. Epub 2016 Mar 16. Expert Opin Investig Drugs. 2016. PMID: 26899229 Review.
-
Olaparib for the treatment of ovarian cancer.Drugs Today (Barc). 2016 Jan;52(1):17-28. doi: 10.1358/dot.2016.52.1.2440714. Drugs Today (Barc). 2016. PMID: 26937492 Review.
Cited by
-
Safety and tolerability of AZD8055 in Japanese patients with advanced solid tumors; a dose-finding phase I study.Invest New Drugs. 2013 Jun;31(3):677-84. doi: 10.1007/s10637-012-9860-4. Epub 2012 Jul 28. Invest New Drugs. 2013. PMID: 22843211 Clinical Trial.
-
Targeted therapy in ovarian cancer.Womens Health (Lond). 2016 Jun;12(3):363-78. doi: 10.2217/whe.16.4. Epub 2016 May 24. Womens Health (Lond). 2016. PMID: 27215391 Free PMC article. Review.
-
Counting the cost of public and philanthropic R&D funding: the case of olaparib.J Pharm Policy Pract. 2022 Aug 16;15(1):47. doi: 10.1186/s40545-022-00445-9. J Pharm Policy Pract. 2022. PMID: 35974344 Free PMC article.
-
Adavosertib in Combination with Olaparib in Patients with Refractory Solid Tumors: An Open-Label, Dose-Finding, and Dose-Expansion Phase Ib Trial.Target Oncol. 2024 Nov;19(6):879-892. doi: 10.1007/s11523-024-01102-8. Epub 2024 Nov 1. Target Oncol. 2024. PMID: 39487373 Free PMC article. Clinical Trial.
-
Olaparib as a rescue treatment in platinum-refractory germ-cell tumors: the IGG-02 phase II trial.ESMO Open. 2025 May;10(5):105056. doi: 10.1016/j.esmoop.2025.105056. Epub 2025 Apr 24. ESMO Open. 2025. PMID: 40279883 Free PMC article. Clinical Trial.
References
-
- Amé J‐C, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays 2004; 26: 882–93. - PubMed
-
- Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 2006; 25: 5864–74. - PubMed
-
- Ashworth A. A synthetic lethal therapeutic approach: Poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double‐strand break repair. J Clin Oncol 2008; 26: 3785–90. - PubMed
-
- Bryant HE, Schultz N, Thomas HD et al. Specific killing of BRCA2‐deficient tumours with inhibitors of poly(ADP‐ribose) polymerase. Nature 2005; 434: 913–7. - PubMed
-
- Farmer H, McCabe N, Lord CJ et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434: 917–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous