A thermostable GH45 endoglucanase from yeast: impact of its atypical multimodularity on activity
- PMID: 22145993
- PMCID: PMC3247070
- DOI: 10.1186/1475-2859-10-103
A thermostable GH45 endoglucanase from yeast: impact of its atypical multimodularity on activity
Abstract
Background: The gene encoding an atypical multi-modular glycoside hydrolase family 45 endoglucanase bearing five different family 1 carbohydrate binding modules (CBM1), designated PpCel45A, was identified in the Pichia pastoris GS115 genome.
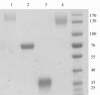
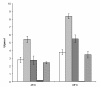
Results: PpCel45A (full-length open reading frame), and three derived constructs comprising (i) the catalytic module with its proximal CBM1, (ii) the catalytic module only, and (iii) the five CBM1 modules without catalytic module, were successfully expressed to high yields (up to 2 grams per litre of culture) in P. pastoris X33. Although the constructs containing the catalytic module displayed similar activities towards a range of glucans, comparison of their biochemical characteristics revealed striking differences. We observed a high thermostability of PpCel45A (Half life time of 6 h at 80°C), which decreased with the removal of CBMs and glycosylated linkers. However, both binding to crystalline cellulose and hydrolysis of crystalline cellulose and cellohexaose were substantially boosted by the presence of one CBM rather than five.
Conclusions: The present study has revealed the specific features of the first characterized endo β-1,4 glucanase from yeast, whose thermostability is promising for biotechnological applications related to the saccharification of lignocellulosic biomass such as consolidated bioprocessing.
Figures



References
-
- Margeot A, Hahn-Hagerdal B, Edlund M, Slade R, Monot F. New improvements for lignocellulosic ethanol. Curr Opin Biotechnol. 2009;20:372–380. - PubMed
-
- Himmel M. Deconstructing the plant cell wall for bioenergy. Blackwell Pub; 2008.
-
- Sánchez C. Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv. 2009;27:185–194. - PubMed
-
- Henrissat B, Driguez H, Viet C, Schülein M. Synergism of Cellulases from Trichoderma reesei in the degradation of cellulose. Nature Biotechnol. 1985;3:722–726.
-
- Harris PV, Welner D, McFarland KC, Re E, Navarro Poulsen JC, Brown K, Salbo R, Ding H, Vlasenko E, Merino S, Xu F, Cherry J, Larsen S, Lo Leggio L. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: structure and function of a large, enigmatic family. Biochemistry. 2010;49:3305–3316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

