Effects of hydrogen-rich water on abnormalities in a SHR.Cg-Leprcp/NDmcr rat - a metabolic syndrome rat model
- PMID: 22146083
- PMCID: PMC3231949
- DOI: 10.1186/2045-9912-1-26
Effects of hydrogen-rich water on abnormalities in a SHR.Cg-Leprcp/NDmcr rat - a metabolic syndrome rat model
Abstract
Background: Hydrogen (H2), a potent free radical scavenger, selectively reduces the hydroxyl radical, which is the most cytotoxic of the reactive oxygen species (ROS). An increase in oxygen free radicals induces oxidative stress, which is known to be involved in the development of metabolic syndrome. Therefore, we investigated whether hydrogen-rich water (HRW) affects metabolic abnormalities in the metabolic syndrome rat model, SHR.Cg-Leprcp/NDmcr (SHR-cp).
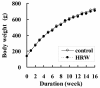
Methods: Male SHR-cp rats (5 weeks old) were divided into 2 groups: an HRW group was given oral HRW for 16 weeks, and a control group was given distilled water. At the end of the experiment, each rat was placed in a metabolic cage for 24 h, fasted for 12 h, and anesthetized; the blood and kidneys were then collected.
Results: Sixteen weeks after HRW administration, the water intake and urine flow measured in the metabolic cages were significantly higher in the HRW group than in the control group. The urinary ratio of albumin to creatinine was significantly lower and creatinine clearance was higher in the HRW group than in the control group. After the 12-h fast, plasma urea nitrogen and creatinine in the HRW group were significantly lower than in the control group. The plasma total antioxidant capacity was significantly higher in the HRW group than in the control group. The glomerulosclerosis score for the HRW group was significantly lower than in the control group, and a significantly positive correlation was observed between this score and plasma urea nitrogen levels.
Conclusion: The present findings suggest that HRW conferred significant benefits against abnormalities in the metabolic syndrome model rats, at least by preventing and ameliorating glomerulosclerosis and creatinine clearance.
Figures


Similar articles
-
Hydrogen-rich water inhibits glucose and α,β -dicarbonyl compound-induced reactive oxygen species production in the SHR.Cg-Leprcp/NDmcr rat kidney.Med Gas Res. 2012 Jul 9;2(1):18. doi: 10.1186/2045-9912-2-18. Med Gas Res. 2012. PMID: 22776773 Free PMC article.
-
Hydrogen improves neurological function through attenuation of blood-brain barrier disruption in spontaneously hypertensive stroke-prone rats.BMC Neurosci. 2015 Apr 20;16:22. doi: 10.1186/s12868-015-0165-3. BMC Neurosci. 2015. PMID: 25925889 Free PMC article.
-
Hydrogen-Rich Water and Lactulose Protect Against Growth Suppression and Oxidative Stress in Female Piglets Fed Fusarium Toxins Contaminated Diets.Toxins (Basel). 2018 Jun 4;10(6):228. doi: 10.3390/toxins10060228. Toxins (Basel). 2018. PMID: 29867031 Free PMC article.
-
Hydrogen-Rich Water to Enhance Exercise Performance: A Review of Effects and Mechanisms.Metabolites. 2024 Oct 7;14(10):537. doi: 10.3390/metabo14100537. Metabolites. 2024. PMID: 39452918 Free PMC article. Review.
-
The Effects of Hydrogen-Rich Water on Blood Lipid Profiles in Clinical Populations: A Systematic Review and Meta-Analysis.Pharmaceuticals (Basel). 2023 Jan 18;16(2):142. doi: 10.3390/ph16020142. Pharmaceuticals (Basel). 2023. PMID: 37259294 Free PMC article. Review.
Cited by
-
Hydrogen treatment: a novel option in liver diseases.Clin Med (Lond). 2021 Mar;21(2):e223-e227. doi: 10.7861/clinmed.2020-0370. Clin Med (Lond). 2021. PMID: 33762390 Free PMC article. Review.
-
Molecular hydrogen in the treatment of acute and chronic neurological conditions: mechanisms of protection and routes of administration.J Clin Biochem Nutr. 2017 Jul;61(1):1-5. doi: 10.3164/jcbn.16-87. Epub 2017 Jun 15. J Clin Biochem Nutr. 2017. PMID: 28751802 Free PMC article. Review.
-
Molecular hydrogen: a preventive and therapeutic medical gas for various diseases.Oncotarget. 2017 Sep 21;8(60):102653-102673. doi: 10.18632/oncotarget.21130. eCollection 2017 Nov 24. Oncotarget. 2017. PMID: 29254278 Free PMC article. Review.
-
Hydrogen-rich saline prevents bone loss in diabetic rats induced by streptozotocin.Int Orthop. 2017 Oct;41(10):2119-2128. doi: 10.1007/s00264-017-3581-4. Epub 2017 Jul 26. Int Orthop. 2017. PMID: 28748382
-
Therapeutic Potential of Molecular Hydrogen in Metabolic Diseases from Bench to Bedside.Pharmaceuticals (Basel). 2023 Apr 4;16(4):541. doi: 10.3390/ph16040541. Pharmaceuticals (Basel). 2023. PMID: 37111299 Free PMC article. Review.
References
-
- Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

