Activin enhances skin tumourigenesis and malignant progression by inducing a pro-tumourigenic immune cell response
- PMID: 22146395
- PMCID: PMC3247817
- DOI: 10.1038/ncomms1585
Activin enhances skin tumourigenesis and malignant progression by inducing a pro-tumourigenic immune cell response
Abstract
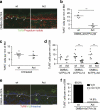
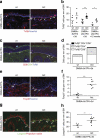
Activin is an important orchestrator of wound repair, but its potential role in skin carcinogenesis has not been addressed. Here we show using different types of genetically modified mice that enhanced levels of activin in the skin promote skin tumour formation and their malignant progression through induction of a pro-tumourigenic microenvironment. This includes accumulation of tumour-promoting Langerhans cells and regulatory T cells in the epidermis. Furthermore, activin inhibits proliferation of tumour-suppressive epidermal γδ T cells, resulting in their progressive loss during tumour promotion. An increase in activin expression was also found in human cutaneous basal and squamous cell carcinomas when compared with control tissue. These findings highlight the parallels between wound healing and cancer, and suggest inhibition of activin action as a promising strategy for the treatment of cancers overexpressing this factor.
Figures





References
-
- Schäfer M. & Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell. Biol. 9, 628–638 (2008). - PubMed
-
- Chen Y. G. et al.. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp. Biol. Med. (Maywood) 231, 534–544 (2006). - PubMed
-
- Werner S. & Alzheimer C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev. 17, 157–171 (2006). - PubMed
-
- Risbridger G. P., Schmitt J. F. & Robertson D. M. Activins and inhibins in endocrine and other tumors. Endocr. Rev. 22, 836–858 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

